Hazard Analysis and Critical Control Points (HACCP) Defined
Farm-to-fork is a concept to describe the control of food safety starting in the fields of a farm and ending with deliciousness in my mouth. The more that is optimized at every step, the more food safety and quality are realized. Farm-to-fork is not a concept reserved for foodies or “eat local” food campaigns and applies to all scales of food manufacture. HACCP is like putting the last piece of a huge puzzle in the middle and seeing the whole picture develop. HACCP is a program to control food safety at the step of food processing. In states where cannabis is legal, the state department of public health or state department of agriculture may require food manufacturers to have a HACCP plan. The HACCP plan is a written document identifying food safety hazards and how those hazards are controlled by the manufacturer. While there are many resources available for writing a HACCP plan, like solving that puzzle, it is a do-it-yourself project. You can’t use someone else’s “puzzle,” and you can’t put the box on a shelf and say you have a “puzzle.”
HACCP is pronounced “ha” as in “hat” plus “sip.”
(Say it aloud.)
3-2-1 We have liftoff.
The history of HACCP starts not with Adam eating in the garden of Eden but with the development of manned missions to the moon, the race to space in the 1950s. Sorry to be gross, but imagine an astronaut with vomiting and diarrhea as a result of foodborne illness. In the 1950s, the food industry relied on finished product testing to determine safety. Testing is destructive of product, and there is no amount of finished product testing that will determine food is safe enough for astronauts. Instead, the food industry built safety into the process. Temperature was monitored and recorded. Acidity measured by pH is an easy test. Rather than waiting to test the finished product in its sealed package, the food industry writes specifications for ingredients, ensures equipment is clean and sanitized, and monitors processing and packaging. HACCP was born first for astronauts and now for everyone.
HACCP is not the only food safety program.
If you are just learning about HACCP, it is a great place to start! There is a big world of food safety programs. HACCP is required by the United States Department of Agriculture for meat processors. The Food and Drug Administration (FDA) requires HACCP for seafood processing and 100% juice manufacture. For all foods beyond meat, seafood and juice, FDA has the Food Safety Modernization Act (FSMA) to enforce food safety. FSMA was signed in 2011 and became enforceable for companies with more than 500 employees in September of 2016; all food companies are under enforcement in September 2018. FSMA requires all food companies with an annual revenue greater than $1 million to follow a written food safety plan. Both FDA inspectors and industry professionals are working to meet the requirements of FSMA. There are also national and international guidelines for food safety with elements of HACCP which do not carry the letter of law.
The first step in HACCP is a hazard analysis.
Traditionally HACCP has focused on processing and packaging. Your organization may call that manufacturing or operations. In a large facility there is metering of ingredients by weight or volume and mixing. A recipe or batch sheet is followed. Most, but not all, products have a kill step where high heat is applied through roasting, baking, frying or canning. The food is sealed in packaging, labeled, boxed and heads out for distribution. For your hazard analysis, you identify the potential hazards that could cause injury or illness, if not controlled during processing. Think about all the potential hazards:
- Biological: What pathogens are you killing in the kill step? What pathogens could get in to the product before packaging is sealed?
- Chemical: Pesticides, industrial chemicals, mycotoxins and allergens are concerns.
- Physical: Evaluate the potential for choking hazards and glass, wood, hard plastic and metal.
The hazards analysis drives everything you do for food safety.
I cannot emphasize too much the importance of the hazard analysis. Every food safety decision is grounded in the hazard analysis. Procedures will be developed and capital will be purchased based on the hazard analysis and control of food safety in your product. There is no one form for the completion of a hazard analysis.
So where do you start? Create a flow diagram naming all the steps in processing and packaging. If your flow diagram starts with Receiving of ingredients, then the next step is Storage of ingredients; include packaging with Receiving and Storage. From Storage, ingredients and packaging are gathered for a batch. Draw out the processing steps in order and through to Packaging. After Packaging, there is finished product Storage and Distribution. Remember HACCP focuses on the processing and packaging steps. It is not necessary to detail each step on the flow diagram, just name the step, e.g. Mixing, Filling, Baking, etc. Other supporting documents have the details of each step.
For every step on the flow diagram, identify hazards.
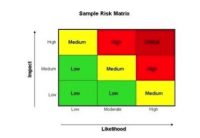
Transfer the name of the step to the hazard analysis form of your choice. Focus on one step at a time. Identify biological, chemical and physical hazards, if any, at that step. The next part is tricky. For each hazard identified, determine the probability of the hazard occurring and severity of illness or injury. Some hazards are easy like allergens. If you have an ingredient that contains an allergen, the probability is high. Because people can die from ingestion of allergens when allergic, the severity is high. Allergens are a hazard you must control. What about pesticides? What is the probability and severity? I can hear you say that you are going to control pesticides through your purchasing agreements. Great! Pesticides are still a hazard to identify in your hazard analysis. What you do about the hazard is up to you.





















 What does this mean for Canada’s largest LP? A strong, multi-country presence in the medical cannabis space that, strategically, is par to none other. There are other Canadian LPs who are planning production facilities in other EU countries of course. And some Canadian companies who appear to see Europe as one giant export market. Germany is just one of them. However, the German-Spanish connection is interesting for several reasons: The two most interesting markets globally right now from both a strictly medical perspective with a clear pathway to much broader acceptance as it transitions into some kind of recreational reform, are Spain and Germany. While the former has not signed up for full-boat medical acceptance, the recent independent assertion by the Catalonian government that they would formalize the cannabis club system is seen here as one more step towards the inevitable. So are ongoing and significant Spanish medical cannabis trials.
What does this mean for Canada’s largest LP? A strong, multi-country presence in the medical cannabis space that, strategically, is par to none other. There are other Canadian LPs who are planning production facilities in other EU countries of course. And some Canadian companies who appear to see Europe as one giant export market. Germany is just one of them. However, the German-Spanish connection is interesting for several reasons: The two most interesting markets globally right now from both a strictly medical perspective with a clear pathway to much broader acceptance as it transitions into some kind of recreational reform, are Spain and Germany. While the former has not signed up for full-boat medical acceptance, the recent independent assertion by the Catalonian government that they would formalize the cannabis club system is seen here as one more step towards the inevitable. So are ongoing and significant Spanish medical cannabis trials.







