As if the cannabis industry doesn’t regularly go through enough rapid change, with COVID-19, cultivators, processors and dispensers of all sizes are trying to do more with less. Lower operational headcount and unpredictable production volume, along with a rapidly-changing supply chain – make eliminating manual steps a necessity. Labels include barcodes or various barcode symbologies to help companies manage inventory, identify products and ultimately ensure that the right products get to the right customers at the right time. By eliminating manual steps in your labeling environment, you can address these issues through automation, scalability, efficiency and accuracy: benefits that will last through the pandemic and position you well for the recovery period.
Automation
Automation of many kinds is being implemented from seed to sale including barcode label printing automation. Integration of labeling software and seed to sale traceability systems including METRC, BioTrack, Leaf Data and others enables streamlined barcode label data population and high-volume label printing to counteract the decreasing operational headcount and eliminate manual touchpoints.
Print automation can be defined as “a centralized technology that replaces the manual process of triggering a print job within a labeling environment.” Look for a labeling software solution that allows you to:
-
- Completely automate your label printing process
- Print to a greater number of printers
- Initiate printing directly from any business system

By integrating your label printing system with your seed to sale traceability system, you can expect to minimize errors, increase print speeds and maximize your ROI. Your business system already holds the variable data such as product names, license number, batch or lot codes, allergens, net quantity, cannabis facts, warning statements and more. By systematically sending this data to the right label template at the right time, labeling becomes an efficient and cost-effective process.
Scalability
One of the most important considerations for cannabis cultivators, processors and dispensers is to invest in solutions that can grow and pivot quickly as the business changes. Whether you are responding to temporary requirements or changes, or your business needs to scale up quickly to respond to a spike in demands as a result of COVID-19 or to prepare for coming out of this pandemic. Whatever your needs are, think about short-term and long-term goals for sustainable business solutions. Scalability includes:
Printing to a greater number of printers
As needs and automation requirements change, and your printer inventory has the possibility of increasing, make sure your labeling design software can be licensed per simultaneous user, with cost-effective, multi-user networking licenses. That way, you don’t run the risk of paying for more printers as you grow or going over budget with each additional printer.
Print documents and labels from the same application
If you use the same data for your documents (like order receipts, bills of materials or packing lists) and labels, moving document printing into your label design software makes sense logistically. An advanced label creation and integration software enables label and document printing standardization by allowing multiple database records to be on one file. That means when new documents or labels come into your database, your software can seamlessly integrate.
Efficiency and accuracy
In a time where responding to the changing market needs to happen very quickly, where costs are being scrutinized and when errors cannot happen – you need to set up your labeling environment to have high levels of accuracy and control. With increased accuracy you will reduce waste, eliminate returns due to mislabeled product, efficiently track product and gain more efficiencies that will save you money and time.
Cutting manual steps out of the process
Removing manual steps in your printing process is a sure-fire way to gain efficiency and accuracy in your labeling environment. Look for labeling software features that allow you to add variable data from a device to your labels automatically, which limits the human interaction with your labels and in turn helps minimize human errors. Other efficiencies include:

- Increased print speed within your labeling environment
- Reduced label waste
- Collection of data from several devices such as:
- Scanners
- Scales
- Keyboards
On-demand color labeling
On-demand labeling is specifically helpful in the cannabis labeling world because of all the regulations you must comply with. Each state has its own regulations, which means each label throughout the cannabis supply chain must be compliant with whichever state they are located. With on-demand labeling, cannabis companies print labels as needed and make changes as they go without the risk of wasting obsolete pre-printed label stock. This is beneficial as pre-printed labels often have large minimum order quantities. On-demand labeling also helps companies maintain better control of their own branding and graphics.
With on-demand labeling, label information can be populated by using pre-approved label templates in order to save you time with the variations of cannabis labels. This gives you the ability to print the specific label you need without having to waste your pre-printed label stock, or spend time switching out your pre-printed label stock in your printer.
Cannabis cultivators, processors and dispensers are faced with many obstacles during these challenging times due to COVID-19 – ensuring workers are safe, keeping operations at 100% capacity with potentially fewer people, creating contingency plans that may be changing daily. In an environment that is changing very quickly, consider how labeling solutions can evolve. You may also need to lean more on your partners than you ever have in the past.
















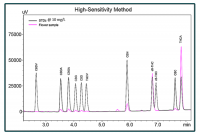




 So what happens if a cannabis lab uses non-certified reference materials? Labs might save money in the short term. CRMs are slightly more expensive than a non-certified reference material, but will increase the defensibility of a lab’s data. Using a reference material created in-house or from a non-accredited vendor can lead to less-than-accurate results. A non-certified reference material has a greater chance of being made incorrectly. The publication of incorrect data damages the credibility of the testing lab and could lead to legal action against the lab from damaged parties.
So what happens if a cannabis lab uses non-certified reference materials? Labs might save money in the short term. CRMs are slightly more expensive than a non-certified reference material, but will increase the defensibility of a lab’s data. Using a reference material created in-house or from a non-accredited vendor can lead to less-than-accurate results. A non-certified reference material has a greater chance of being made incorrectly. The publication of incorrect data damages the credibility of the testing lab and could lead to legal action against the lab from damaged parties.


