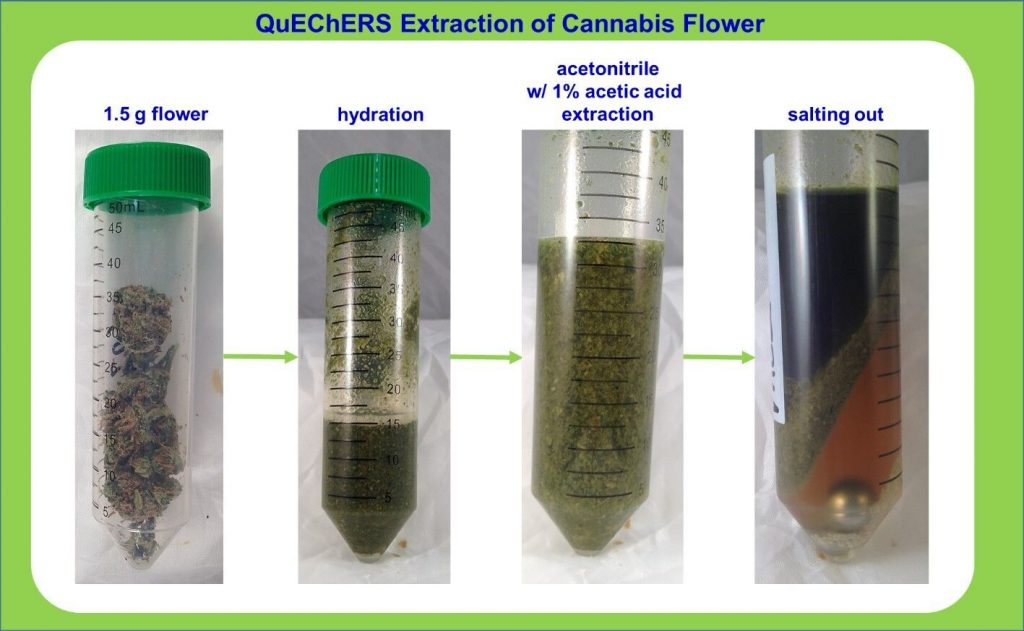
Last Friday, the Bureau of Marijuana Control, the regulatory body overseeing California’s cannabis industry, released a set of proposed regulations for the lab testing market. The regulations are somewhat comprehensive, covering sampling, licensing, pesticide testing, microbiological contaminants, residual solvents, water activity and much more.
Formerly named the Bureau of Medical Cannabis Regulation under the state’s Department of Consumer Affairs, the Bureau of Marijuana Control is tasked with overseeing the development, implementation and enforcement of the regulations for the state’s cannabis industry. In their statement of reasons for the lab testing regulations, the bureau says they are designed with public health and safety at top of mind. At first glance, much of these laboratory rules seem loosely modeled off of Colorado and Oregon’s already implemented testing regulations.
The regulations lay out requirements for testing cannabis products prior to bringing them to market. That includes testing for residual solvents and processing chemicals, microbiological contaminants, mycotoxins, foreign materials, heavy metals, pesticides, homogeneity as well as potency in quantifying cannabinoids.
The microbiological impurities section lays out some testing requirements designed to prevent food-borne illness. Labs are required to test for E. coli, Salmonella and multiple species of the pathogenic Aspergillus. If a lab detects any of those contaminants, that batch of cannabis or cannabis products would then fail the test and could not be sold to consumers. A lab must report all of that information on a certificate of analysis, according to the text of the regulations.
The proposed regulations stipulate requirements for sampling, including requiring labs to develop sampling plans with standard operating procedures (SOPs) and requiring a lab-approved sampler to follow chain-of-custody protocols. The rules also propose requiring SOPs for analytical methodology. That includes some method development parameters like the list of analytes and applicable matrices. It also says all testing methods need to be validated and labs need to incorporate guidelines from the FDA’s Bacterial Analytical Manual, the U.S. Pharmacopeia and AOAC’s Official Methods of Analysis for Contaminant Testing, or other scientifically valid testing methodology.
Labs will be required to be ISO 17025-accredited in order to perform routine cannabis testing. Laboratories also need to participate in proficiency testing (PT) program “provided by an ISO 17043 accredited proficiency-test provider.” If a laboratory fails to participate in the PT program or fails to pass to receive a passing grade, that lab may be subject to disciplinary action against the lab’s license. Labs need to have corrective action plans in place if they fail to get a passing grade for any portion of the PT program.



















 So what happens if a cannabis lab uses non-certified reference materials? Labs might save money in the short term. CRMs are slightly more expensive than a non-certified reference material, but will increase the defensibility of a lab’s data. Using a reference material created in-house or from a non-accredited vendor can lead to less-than-accurate results. A non-certified reference material has a greater chance of being made incorrectly. The publication of incorrect data damages the credibility of the testing lab and could lead to legal action against the lab from damaged parties.
So what happens if a cannabis lab uses non-certified reference materials? Labs might save money in the short term. CRMs are slightly more expensive than a non-certified reference material, but will increase the defensibility of a lab’s data. Using a reference material created in-house or from a non-accredited vendor can lead to less-than-accurate results. A non-certified reference material has a greater chance of being made incorrectly. The publication of incorrect data damages the credibility of the testing lab and could lead to legal action against the lab from damaged parties.