The basis of anecdotal controversy continues about the use of hydrocarbons versus carbon dioxide. It is important to note that hydrocarbons span a range of phases on the planet earth.
It is important to eliminate the cost of the instruments and the cost of the facilities from this comparison to keep the discussion on specifically the extraction principles.
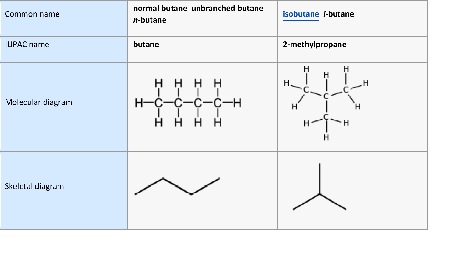
Butane is a gaseous hydrocarbon. As you add more carbons to hydrocarbons, they move from gaseous to liquid.
It is also important to note that the same is true of carbon dioxide in its natural form on the earth’s atmosphere, it is a gas. It is nonflammable and used in fire extinguishers.
At typical conditions, carbon dioxide in the supercritical range is similar to hexane (C6H14) and ethyl acetate in its solubility characteristics. Propane (C3H8) and butane (C4H10) are gases at normal atmospheric conditions. Both must be manipulated for the extraction of CBDA and CBD. For example, both CO2 and C4H10 must be placed under pressure and then passed through the material to extract the lipophilic terpenes and cannabinoids.
For this short discussion, let’s remove the concern about the different volatilities of the compounds. Hydrocarbons with a spark will be significantly more powerful of an explosion than carbon dioxide (note it could be used to put out the butane fire). The hydrocarbons can be in more configurations and therefore the getting the correct form initially is critical. For example, butane can have all the carbons in a row like a train, or branched like a tree. Those are very different and have different characteristics too. Getting pharmaceutical grade butane is essential to ensure safety. The concern that people have expressed with butane is what is in the other 0.1% for 99.9%. Checking for residual butane is less of a concern than the polyaromatic hydrocarbons in the untested cylinder. Furthermore, in the wrong hands it can be more volatile.
The critical premise that needs to be considered is the final formulation. Is one solvent significantly more applicable than the other? No. They have different characteristics.
Propane is a common solvent in the spices, flavors and fragrances industry. For example, the extraction of lipids and oils from vegetables and the fatty oils from seeds, it would be an advantage to have a solvent that is totally miscible, i.e. will be totally soluble in a fluid. This is similar to the idea of sugar in hot water versus in water in ice. If an example of cardamom were used comparing CO2 and propane (which is similar to butane), the pressure needed for CO2 would be 100 bar, while propane would be only 20 bar. However the increasing the pressure of the propane from 20 to 50 bar at a constant 25 C, also increases the chlorophyll from 3.4 g/g oil to 10.8 g/g oil. Meanwhile with the more finely tunable CO2 from 80 to 100 to 200 the amount of chlorophyll is negligible (0.36 g/g oil) but at 300 bar it dramatically increases to 4.53 g/g oil.
Additionally the CO2 is a better extraction for the terpenes in the cardamom. The beta-pinine, Cineole, linalool, alpha-terpinol and bornelole. The increase in the propane pressure will allow us to increase the yield of the CO2 (Illes, V, et. al. Proceedings of the Fifth Meeting of Supercritical Fluids, Nice, France, Tome 2, 555-560).
This example is the same with the butane and cannabis. Butane is a stronger solvent and if left too long will continue to pull out more and more polar compounds like chlorophyll. With the fine-tuning of CO2, you can eliminate or you can pull out the chlorophyll if you choose the wrong conditions.
So fast extractions are possible with butane but little control of all the material, while CO2 can be tunable and therefore is able to collect all of the same material, just through a segmented process.



