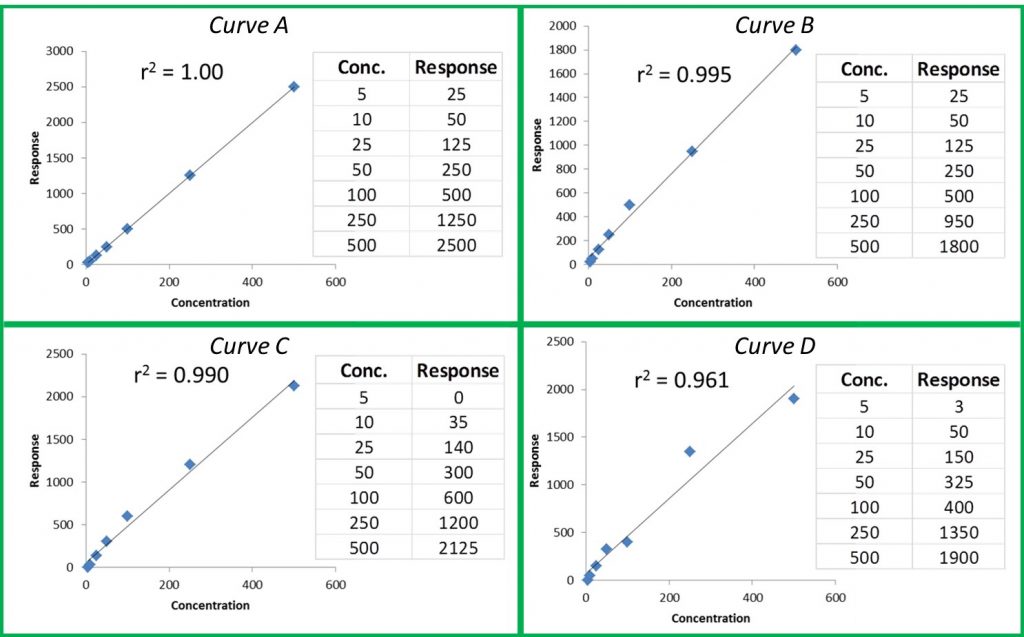
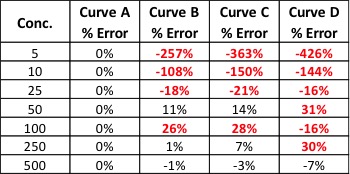
The American Association for Laboratory Accreditation (A2LA) and Americans for Safe Access (ASA) announced yesterday a collaboration to develop a cannabis-specific laboratory accreditation program based upon the requirements of ISO/IEC 17025 and ASA’ s Patient Focused Certification (PFC) Program. Accreditation under this program will offer the highest level of recognition and provide the most value to the laboratory and users of the products tested, according to a press release published yesterday. ASA is the largest medical cannabis patient advocacy group in the United States. “A2LA is pleased to partner with ASA to offer a cannabis testing laboratory accreditation program to ISO/IEC 17025 as well as the additional laboratory requirements from ASA’s Patient Focused Certification Program,” says Roger Brauninger, biosafety program manager at A2LA.
under this program will offer the highest level of recognition and provide the most value to the laboratory and users of the products tested, according to a press release published yesterday. ASA is the largest medical cannabis patient advocacy group in the United States. “A2LA is pleased to partner with ASA to offer a cannabis testing laboratory accreditation program to ISO/IEC 17025 as well as the additional laboratory requirements from ASA’s Patient Focused Certification Program,” says Roger Brauninger, biosafety program manager at A2LA.

Jahan Marcu, Ph.D Chief Scientist, PFC at ASA;
Roger Brauninger, BioSafety Program Manager, A2LA;
Michelle Bradac, Senior Accreditation Officer, A2LA
The program affirms that cannabis laboratories are compliant with state and local regulations and ensures that they adhere to the same standards that are followed by laboratories used and inspected by the Environmental Protection Agency (EPA), the United States Department of Agriculture (USDA), and the U.S. Consumer Product Safety Commission (CPSC) among other regulatory bodies. The two non-profit organizations will offer their first joint training course at A2LA’s headquarters in Maryland from July 11th to the 15th. During this course, participants will receive training on PFC’s national standards for the cultivation, manufacture, dispensing, and testing of cannabis and cannabis products, combined with ISO/IEC 17025 training.
The guidelines for cannabis operations that serve as the basis for this accreditation program were issued by the American Herbal Products Association (AHPA) Cannabis Committee, an industry stakeholder panel, and have already been adopted by sixteen states. “We are very excited to see the PFC program join the ISO/IEC 17025 accreditation efforts to help fully establish a robust and reliable cannabis testing foundation,” says Jeffrey Raber, chief executive officer of The Werc Shop, a PFC-certified cannabis testing laboratory. “It is a great testament to ASA’s commitment to quality in their PFC program by partnering with a world-renowned accrediting body to set a new standard for cannabis testing labs.”
According to Kristin Nevedal, program director of PFC, this is an important first in the industry. “This new, comprehensive accreditation program affirms laboratory operations are meeting existing standards and best practices, adhering to the ISO/IEC 17025 criteria, and are compliant with state and local regulations,” says Nevedal. “This program is the first of its kind developed specifically for the cannabis industry, giving confidence to patients as well as regulators that their test results on these products are accurate and consistent.”
“The program will combine the expertise and resources of the country’s largest accreditation body with the scientific rigor and knowledge base of the nation’s largest medical cannabis advocacy group, benefitting the myriad of laboratories tasked with analyzing cannabis products,” says Nevedal. According to Brauninger, a cannabis-specific accreditation program is vital to the industry’s constantly shifting needs. “The ability to now offer a cannabis testing laboratory accreditation program that is tailored to address the unique concerns and issues of the industry will help to add the necessary confidence and trust in the reliability and safety of the cannabis products on the market,” says Brauninger. “Those laboratories that gain accreditation under this program will be demonstrating that they adhere to the most comprehensive and relevant set of criteria by their compliance to both the underlying framework of the internationally recognized ISO/IEC 17025:2005 quality management system standard and the specific guidelines issued by the AHPA Cannabis Committee.” This type of collaboration could represent a milestone in progress toward achieving a higher level of consumer safety in the cannabis industry.