Back in November of 2017, urban-gro announced the development of their Soleil Technologies platform, the first technology line for cannabis growers utilizing Internet-of-Things (IoT). Today, urban-gro is announcing that line is now officially available.

The technology portfolio, aimed at larger, commercial-scale growers, is essentially a network of monitors, sensors and controls that give cultivators real-time data on things like temperature, humidity, light, barometric pressure and other key factors. The idea of using IoT and hypersensitive monitoring is not new to horticulture, food or agriculture, but this is certainly a very new development for the cannabis growing space.

According to Brad Nattrass, chief executive officer and co-founder of urban-gro, it’s technology like this that’ll help growers control microclimates, helping them make the minor adjustments needed to ultimately improve yield and quality. “As ROI and optimized yields become increasingly important for commercial cultivators, the need for technologies that deliver rich granular data and real-time insights becomes critical,” says Nattrass. “With the ability to comprehensively sense, monitor, and control the microclimates throughout your facility in real-time, cultivators will be able to make proactive decisions to maximize yields.”

One of the more exciting aspects of this platform is the integration of sensors, and controls with automation. With the system monitoring and controlling fertigation, lighting and climate, it can detect when conditions are not ideal, which gives a cultivator valuable insights for directing pest management or HVAC decisions, according to Dan Droller, vice president of corporate development with urban-gro. “As we add more data, for example, adding alerts for when temperatures falls or humidity spikes can tell a grower to be on the lookout for powdery mildew,” says Droller. “We saw a corner of a bench get hot in the system’s monitoring, based on predefined alerts, which told us a bench fan was broken.” Hooking up a lot of these nodes and sensors with IoT and their platform allows the grower to get real-time monitoring on the entire operation, from anywhere with an Internet connection.
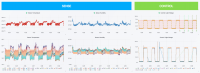
Droller says using more and more sensors creates super high-density data, which translates to being able to see a problem quickly and regroup on the fly. “Cannabis growers need to maintain ideal conditions, usually they do that with a handful of sensors right now,” says Droller. “They get peace of mind based on two or three sensors sending data points back. Our technology scales to the plant and bench level, connecting all of the aggregate data in one automated system.”
In the future, urban-gro is anticipating this will lay the groundwork for using artificial intelligence to learn when controls need to be adjusted based on the monitoring. Droller hopes to see the data from environmental conditions mapped with yield and by strain type, which could allow for ultra-precise breeding based on environmental conditions. “As we add more and more data and develop the platform further, we can deliver some elements of AI in the future, with increased controls and more scientific data,” says Droller.