In any industry that is labeled as the “Wild West,” the ability to discern your target market, collect accurate data and strategically drive success has become paramount. With factors such as invisible shoppers, anyone who is a customer of a brand or dispensary and does not personally purchase what they consume, it’s critical to understand how and where the data an organization is using to make decisions is sourced. Effective market research is the cornerstone upon which cannabis brands can build their trajectory to growth, armed with a diverse array of methodologies designed to provide insightful data-driven insights. Through leveraging the power of dependable data, organizations can navigate the intricacies of the cannabis industry across a wide variety of markets, develop solutions that address actual pain points and position themselves as leaders in their niche.
Not to mention, in an industry where competition is fierce and resources have become increasingly scarce, market research emerges as the North Star guiding businesses along their decision-making path. The era of relying on intuition and gut feelings alone is behind us. As this industry matures, making decisions based on inadequate syndicated data has proven detrimental. This is where robust market research steps in, instilling confidence in decision-makers by offering comprehensive and intelligent data for well-informed and defensible choices. Market research naturally falls into two overarching categories: qualitative and quantitative methodologies. Within these categories, various research approaches can be employed to collect actionable insights across the industry.

Qualitative research delves deep into the intricacies of human behavior, motivations and preferences. Techniques like in-depth interviews and focus groups—whether conducted virtually or in person—unearth nuanced insights that transcend mere numerical data. This type of research is particularly invaluable for discerning the human side of B2B interactions within the cannabis industry.
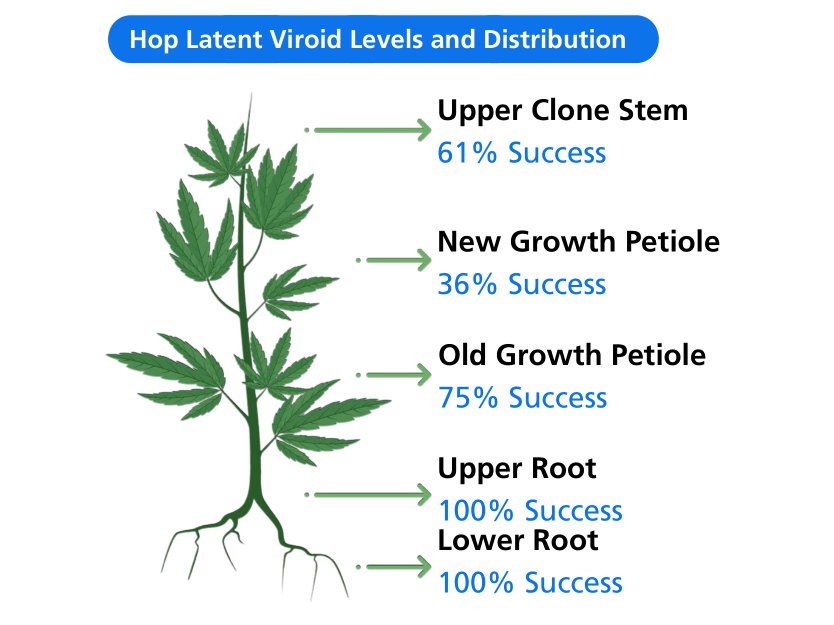
On the other hand, quantitative research focuses on the collection and analysis of numerical data. Through surveys, questionnaires and statistical analysis, companies can glean valuable insights into demographics, preferences and market trends. This data can be powerfully visualized through charts, tables and infographics, providing a clear picture of the market landscape.
A wide spectrum of methodologies can be put in place to garner actionable insights across various aspects:
Precision Product Testing: This methodology empowers businesses to amass authentic feedback regarding product quality, user experience and overall satisfaction. By employing a hybrid approach of qualitative and quantitative methods, companies can refine and develop their offerings, ensuring alignment with the expectations of customers. This approach ensures not only a product’s efficacy, but also its appeal in the context of the consumer’s interaction with the product.
In-Home Usage Tests (IHUTs): IHUTs involve providing cannabis products to customers for testing within their operational or natural environments. Often conducted as “diary studies” over a designated time frame, IHUTs provide invaluable insights into aspects like usability, practicality and long-term effects. For instance, conducting an IHUT study on cannabis-infused products designed to aid sleep can assess factors like taste, texture and efficacy, thereby tailoring products to meet specific needs.
Hands-On Collaboration Sessions: Site-based, central location or mobile product testing enables real-time observation of product trials, enabling an immediate evaluation of sensory attributes and practical effects. These collaborative sessions can involve consumers directly engaging with the products, such as the scenario where consumers grind, roll and smoke their joints. This hands-on approach fosters deeper understanding and involvement, leading to more actionable insights for product enhancement.
Strategic Online Surveys: While online surveys are a well-established approach for capturing quantitative data from a broad consumer base, by designing targeted questionnaires tailored for specific market segments, companies can assess preferences, satisfaction levels, brand perceptions, and even purchasing intentions. These insights are invaluable in engineering products and services to specific consumer needs.

Nuanced Focus Groups (FGs): Focus groups bring together a small group of individuals for facilitated discussions. These discussions can be conducted in a central location or even virtually, allowing geographically diverse participants to contribute insights. This approach is perfect for delving into perceptions, motivations and pain points. For instance, a focus group centered on testing prototypes for cannabis-related accessories can provide valuable input for refining products catering to specific needs.
Tailored In-Depth Interviews (IDIs): In-depth interviews, conducted either in person or virtually, provide an opportunity for one-on-one engagements. These interviews are particularly useful for exploring sensitive or controversial topics. For example, assessing the suitability of a prototype product for budtenders through in-depth interviews ensures candid feedback without the influence of group dynamics.
When embarking on cannabis market research, it’s imperative to navigate the intricate landscape of state regulations, especially when dealing with THC-based products. The cannabis industry operates within a patchwork of regulations, which vary from state to state, impacting the feasibility and logistics of product testing. Adhering to and understanding these regulations ensures compliant product testing, upholds participant safety and generates indispensable insights for product development.
At the end of the day, market research is actively emerging as the linchpin for the short- and long-term prosperity of our industry. Leveraging a blend of qualitative and quantitative approaches, such as surveys, focus groups and product testing, equips businesses with a profound understanding of preferences, motivations, and feedback. By embracing data-driven decision-making, cannabis companies position themselves at the vanguard of data-driven achievement, fortified with confidence and assurance.