Making Cannabis Transparent: The Future of the Industry is Information and Data
The last decade has been marked by great strides in the cannabis industry, as public awareness surrounding the health benefits of marijuana-infused products has spread and products have become increasingly well researched and scientifically advanced. Despite this significant progress, however, cannabis legislation and regulations continue to vary widely between states, ultimately contributing to a lack of clarity within the industry.
This issue was at the forefront of the DispensaryNext Conference and Expo agenda held in Denver a few weeks ago. During the expo’s Consumer Safety and Education discussion, a panel of industry leaders including Kevin Gallagher, director of compliance and government affairs at Craft Concentrates and executive director of the Cannabis Business Alliance (CBA); Eileen Konieczny, registered nurse and president of the American Cannabis Nurses Association; Kevin Staunton, director of business development at RM3 labs; and moderator David Kotler, a partner at Cohen Kotler P.A., highlighted a number of important issues for cannabis patients and adult-use consumers, as well as what’s next for physicians, testing labs and dispensaries across the industry. A number of common themes resonated in their discussion of opportunities and challenges, ultimately pointing to a need for increased research and data, and most notably, a growing demand for transparency industry-wide.
Medical practitioners and dispensary technicians need qualified and legitimate information.
Konieczny opened by stressing that the industry must stop calling dispensary sales associates “budtenders.” “I prefer the term ‘dispensary technician.’ These are knowledgeable people who are on the front lines, helping patients understand the products available to them. They deserve a title to reflect that our industry and their knowledge is much more than ‘bud,’” says Konieczny.
These are knowledgeable people who are on the front lines, helping patients understand the products available to them. The most prominent information gaps in the industry lie at the level of dispensary technicians and medical practitioners. The ideal scenario for patients who are looking to use cannabis as medicine is that their medical practitioner is educated about the endocannabinoid system and that the products are available locally so that a treatment plan can be developed based on their needs. But the reality is that many patients enter their local dispensary without much knowledge or support at all, relying on the professionalism of the dispensary staff to help them navigate the dizzying array of products.
Putting the patient’s safety and success first, it is imperative that everyone involved has the proper data and information to make the best choices. However, dispensary technicians should be extremely careful to avoid making health or benefit claims. As Gallagher noted, “It is not only illegal, but also unethical to make medical claims as a dispenser. There is a difference between a claim and a personal experience. A dispenser can tell their customer that a certain strain helped them personally, but they cannot tell the customer that the strain will cure their specific ailment.”
The industry needs transparency.
New cannabis consumers may have a certain degree of misunderstanding of the products they are consuming and unfortunately, manufacturers do not offer a high level of transparency in disclosing ingredients, thereby preventing these customers from becoming better informed.
While educating the public is essential, educating the industry is of equal importance.Furthermore, labels often contain small barely legible type, along with confusing and unnecessary content. According to Gallagher, the labels need to be simpler. “Products are overloaded with redundant, confusing language that most consumers don’t understand. This turns them off—especially if they’re inexperienced in this realm,” says Gallagher. When customers who are new to cannabis find products off-putting, it hurts not only the industry, but also their own health. Ill-informed consumers may have trouble understanding how cannabis can help them, and therefore they can miss the benefits it provides.
While these issues are prevalent, there are many ways they can be resolved—with transparency at the core.
Research is critical and paramount.
For cultivators or manufacturers, research and data hold the key to attracting new consumers. By providing details about what is in a product and implementing certifications to show the product is contaminant-free, manufacturers are able to provide transparency and offer differentiation.
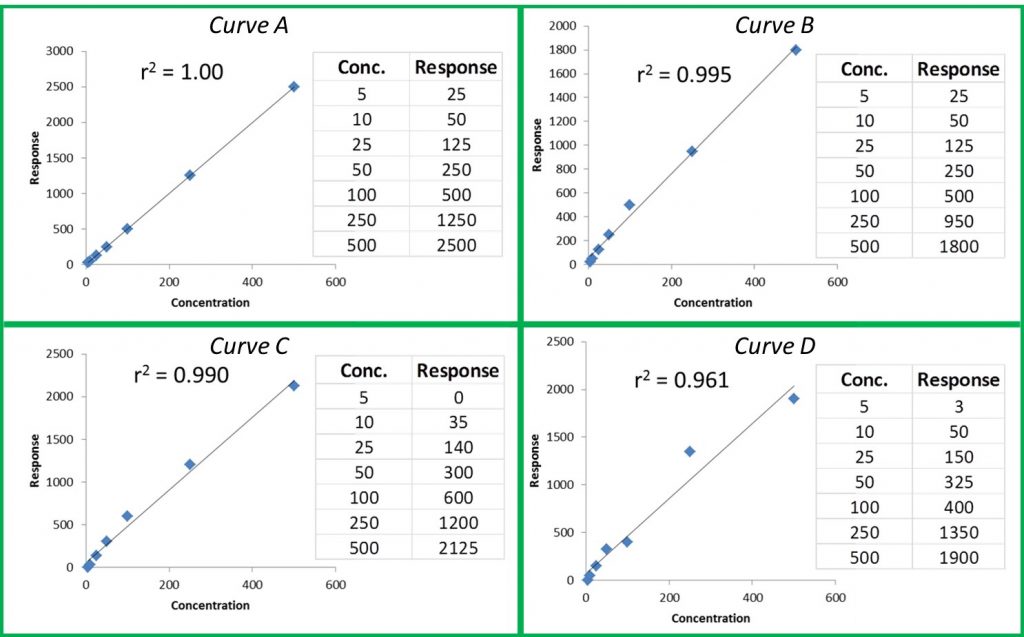
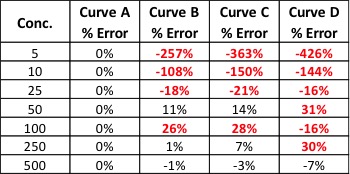
During the panel, Konieczny pointed out another common mistake that many manufacturers make—not sharing test results. “Not many are posting their test results, and yet this is one of the leading avenues that can increase revenue,” says Konieczny. “Most people just want to feel well again, so providing test results adds a layer of legitimacy for patients who are wary to try a new product.”
With all of this in mind, it is perhaps most important to consider the way that this information is conveyed. Facts and research are useless if they are not accessible to consumers, who may not comprehend complex data. “We need to present information in plain language, keeping it clear and simple to understand,” expressed Konieczny. The simpler the delivery, the better it will be understood and knowledge is a very powerful tool for patients, consumers and the bottom line.
Educating the educators.
While educating the public is essential, educating the industry is of equal importance. For instance, thoroughly training dispensary technicians to ask the correct questions and identify first-time users will ensure consumer safety while avoiding improper use.
The industry as a whole depends on transparencyEducating professionals on better product labeling is another critical way that the industry is working to improve itself. There has recently been a push at the manufacturing level for standardization in product labeling, as establishing a clear standard can aid customers in successfully using cannabis. “In working groups with Colorado’s MED (Marijuana Enforcement Division), we aim to standardize specific product categories, remove irrelevant names, and harmonize medical and retail labeling regulations,” says Gallagher. “Ultimately, we want to consolidate language and make it more transparent in promoting public health and safety so that it can be easily read and understood.”
All panelists agreed transparency is paramount for the future of the cannabis industry and for growing a brand. Using lab data can provide value, setting a brand apart and building loyalty among consumers looking for someone they can trust.
“Transparency is king,” Gallagher urges. “The more we educate consumers and professionals, the more clarity we will see at all levels, ultimately minimizing risk and creating greater demand among those consumers. The industry as a whole depends on transparency.”