Now that Canada finally has a date for the recreational market start, the federal government, provinces and other regulatory authorities are beginning to issue guidelines and rules that are going to define the early days of the recreational industry.
These include regulations on retail trade, medical sales and use. However this is precisely where the confusion is growing.
The Government Will Continue To Run The Medical Cannabis System
In a move to protect patients, Health Canada has announced that it will continue to run the medical part of the market for at least the next five years. In good news for medical users, this announcement was made against calls from the Canadian Medical Association for the medical infrastructure developed on Canada’s path to recreational reform to be phased out. The reason, according to the CMA? Many doctors feel uncomfortable prescribing the drug because of a lack of research and a general lack of understanding about dosing.
Both patients and advocates have expressed support for continuing the medical system. This includes organizations like the Canadian Nurses Association who fear that if a focus is taken off of medical use, producers will ignore this part of the market to focus only on recreational sales.
In the future, after legalization, Health Canada will also continue to support more research and trials.
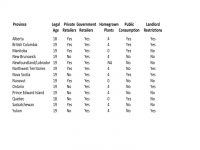
Provinces Are Setting Their Own Rules For Recreational Sales
Despite early statements, the recreational market is still in the throes of market creation and regulation. The laws are also changing in progress, a situation one regulator has described as building an airplane as it hurtles down the runway for take-off.
Athletes in Canada are still banned from using any kind of cannabis.For example, Ontario, the largest provincial market, is also delaying private sector sales in retail shops until next year. It is also moving away from a government-run dispensary model. Government sales will begin in October, but private dispensaries will have to wait until next April to open their doors (and existing operations will have to close their doors while they apply for licenses). This is also a reversal of the regional government’s position that it would only allow government-controlled shops to sell recreational cannabis.
But perhaps the largest unknown in both national and provincial policy outside of retail brick and mortars is in the area of online sales. A major fight is now brewing in many places where the established industry is now siding with the government about unregistered dispensaries (see Ontario) and established if not registered producers are competing directly with the government not only on main street but online as well.recreational users are beginning to sound alarms that they do not want the government to have so much personal information about them
Retailers with a web presence operating in a grey space will continue to pose a significant challenge to the online system now being implemented by the government for two reasons. Product availability (which will be far more limited on the government-run sites) and privacy.
Beyond the lack of diverse products and strains to be initially offered via the online government portals, recreational users are beginning to sound alarms that they do not want the government to have so much personal information about them – and point specifically to the differences in the regulated alcohol industry vs. the new regulations for the recreational cannabis market.
Beyond Market Rules, There Are Other Guidelines Coming
The Canadian military has now issued guidelines for active duty personnel and cannabis. It cannot ban it from soldiers entirely of course, and as it stands, the situation will be ripe for misunderstandings. For example, soldiers are prohibited from consuming cannabis 8 hours before any kind of duty, 24 hours before the operation of any kind of vehicle or weapon and 28 days before parachuting or serving on a military aircraft.
The only problem, of course, is being able to enforce the same. Cannabinoids, notably THC, can stay in the body for up to 30 days for casual users long after the high is over.
Athletes in Canada are still banned from using any kind of cannabis. The reason? They are subject to the Canadian Anti-Doping Program (CADP) under which the use of cannabis will still be prohibited.
That said, the Canadian Hockey League is reportedly now examining how to revise how it addresses the issue of medical use.