Living in a world of ever-increasing interdependence and an era of limited state government, financial and human resources, it is imperative that those charged with protecting the health and safety of patients and users of medical and recreational cannabis products leverage what private sector institutions and existing frameworks already offer in setting up quality standards for laboratory testing operations. That this moment arrives now – at a point where state governments are being tasked with undertaking the most significant change in the regulation of this substance whilst the federal government appears unwilling to play a substantial role – makes this partnership both inevitable and absolutely necessary.
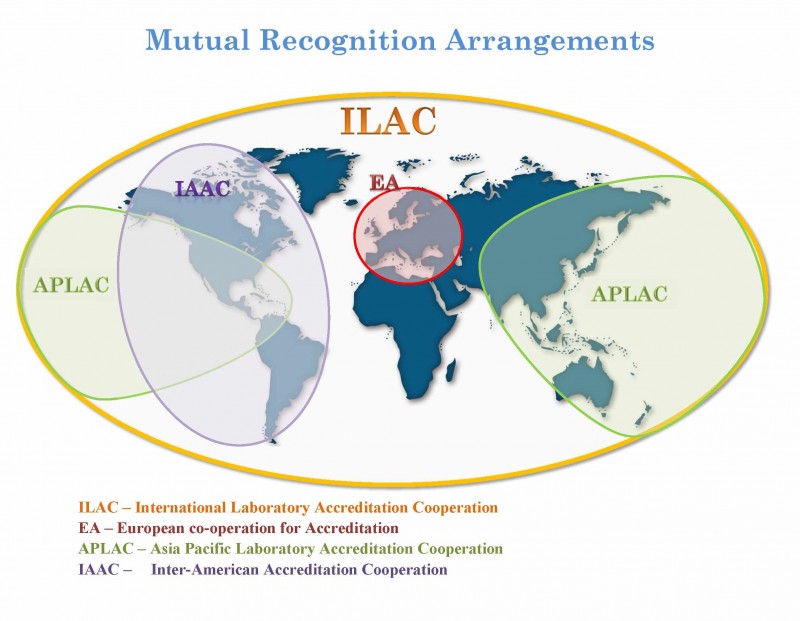
Accreditation is an internationally accepted conformity assessment tool for ensuring laboratory competence and confidence in the accuracy and reliability test data. The accreditation infrastructure is well-established through accreditation bodies (ABs) and the Mutual Recognition Arrangement (MRA) of the International Laboratory Accreditation Cooperation (ILAC), supported by regional cooperative arrangements, including those of the Asia Pacific Laboratory Accreditation Cooperation (APLAC) and the Inter-American Accreditation Cooperation (IAAC). ILAC functions as a forum for harmonization of laboratory accreditation procedures and policies, thus reducing technical barriers to trade and promoting laboratory accreditation as a mechanism for establishing confidence in testing facilities. ILAC MRA signatory ABs are recognized, through a rigorous peer evaluation process, as competent to accredit testing organizations. All signatory ABs must meet the requirements of ISO/IEC 17011 and use ISO/IEC 17025 as the basis for accreditation of laboratories. In turn, under the ILAC MRA and the regional co-operations, competent laboratories are recognized globally, thus facilitating acceptance of the test results that accompany goods across international borders.
In other areas, such as the food supply and energy, both state and federal government have been an active participant in accreditation activities. According to The Administrative Conference of the United States in its Agency Use of Third-Party Programs to Assess Regulatory Compliance*, “…agencies in diverse areas of regulation have developed third-party programs to assess whether regulated entities are in compliance with regulatory standards and other requirements. Through these programs, third parties are charged with assessing the safety of imported food… Third parties also ensure that products labeled as organic and energy-efficient meet applicable federal standards. In these regulatory third-party programs, regulated entities generally contract with third parties to carry out product testing and other regulatory compliance assessment activities in the place of regulatory agencies. Regulatory agencies take on new roles in coordinating and overseeing these third-party actors.” While this reference largely deals with areas outside of cannabis regulation, it remains useful and relevant because of the manner in which cannabis products are used.
Traditionally, ABs have worked with regulators to establish specific technical requirements to supplement the ISO/IEC 17025 accreditation framework. In this partnership, the AB is responsible for executing the assessment and accreditation process but the regulator retains responsibility for the ultimate decisions on the acceptance of that organization’s accreditation. In the example of our food supply and its various sources, governmental recognition of accreditation bodies operating in accordance with international standards is much more practical than government agencies themselves accrediting the individual testing organizations or ABs. Thus the public/private partnership paradigm: To assess regulatory compliance a Regulatory Agency approves ABs that accredit organizations that assess whether Regulated Entities or Regulated Products are in conformity with a Regulatory Standard.*
In this example, all of the organizations are treated equally by the regulatory agency since they use the same recognition criteria for ABs and the same accreditation requirements in the assessment of conformity assessment bodies. This approach would also provide consistency at a point in time where many states are grappling with trying to find the best quality standard to use and which, to date, has resulted in many different standards being chosen or considered for implementation. This is especially true when one looks at the requirements put into place by the “early adopter” states. However, in those states that have entered this area more recently, it seems clear that the consensus is use of ISO/IEC 17025 as the most appropriate quality management standard for testing laboratories.