Cannabis testing laboratories are one of the major players in the industry for protecting public health. Ensuring that laboratory test results are reliable and valid requires a multipronged approach involving method validation, proficiency testing and performing frequent reviews of equipment and processes.
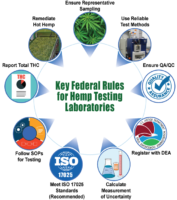
Cannabis testing laboratories often use a variety of different methods to conduct proficiency testing. Laboratories can either participate in programs run by ISO/IEC 17043-accedited proficiency testing providers or through intralaboratory comparison. Comparing different instruments, methods, technologies against pre-defined criteria is a must when validating methods for a specific type of test and ensuring the competence of the laboratory.
Beyond proficiency testing, there are a number of other stopgaps at a laboratory’s disposal for ensuring valid results, like using accredited certified reference materials, performing checks on measuring equipment frequently, reviewing reported results and retesting retained items. All of that and more is outlined in the ISO/IEC 17025:2017 standard, section 7.7.

There’s a lot that goes into making sure laboratories provide valid results, much of which is detailed in the accreditation process. For more information, we sit down with Keith Klemm, senior accreditation manager for ANSI National Accreditation Board to learn about laboratory accreditation, method validation and other certifications and credentialing available in the cannabis industry.
Q: Why is method validation important for cannabis test methods?
Keith Klemm: Because cannabis production, testing, and sales is regulated by each individual state, there are very few standard methods for testing cannabis and cannabis-derived products. Non-standard methods or methods developed by the laboratory must be validated to ensure the methods are fit for their intended purpose. What good is a test result if you cannot attest to its validity? There would be no confidence that the results are accurate. Additionally, while organizations such as ISO, AOAC and ASTM are developing standard methods for use in the laboratory, the wide range of products and matrices being tested require modifications to standard methods. Standard methods used outside their intended scope must also be validated, again to ensure the method remains fit for the intended purpose.
Q: We’re pretty familiar with laboratory accreditation. What other accreditations are available in the cannabis industry?
Klemm: Accreditation programs are available for product certification and personnel credentialing, in addition to laboratory accreditation. ANAB’s product certification program was launched in 2020 and is based on the requirements of ISO/IEC 17065. The program combines the requirements of this standard with specific scheme requirements to attest to the competency of certification bodies who then certify products within the scheme. Two schemes are in development specific to the cannabis industry: Cannabis Safety and Quality (CSQ) and PurityIQ. For personnel credentialing, a new Cannabis Certificate Accreditation Program (C-CAP) was developed and is based on ASTM D8403, Standard Practice for Certificate Programs within the Cannabis and Hemp Industries. It also includes any additional state Responsible Vendor Training requirements.
Q: What are the steps to becoming an accredited cannabis testing laboratory, product certification body, or C-CAP organization?
Klemm: The process begins with a request for quote. The organization prepares for the initial assessment by implementing the requirements of the applicable standards, regulatory requirements, and scheme requirements. ANAB believes in a partnership approach to accreditation with a focus on customer needs while ensuring accreditation requirements are met. Once the organization is ready, an initial document review is performed. The accreditation assessment is then performed on-site by technically skilled and knowledgeable assessors. If any nonconformities are encountered, the organization provides a response with cause and corrective actions. Once all nonconformities are resolved and technical review is completed, a scope of accreditation and certificate are provided to the organization. The technical review may vary depending on the accreditation that is being sought, but the general process of accreditation is the same. After accreditation is achieved, the organization moves into a cycle of surveillance and reassessment as defined by the accreditation program and any scheme requirements.
 About Keith Klemm
About Keith Klemm
Keith Klemm is a graduate of Manchester University with a B.S. in Biology. Keith is an experience laboratory director and operations manager with 30 years’ experience in the laboratory environment and has worked as a senior accreditation manager for ANSI National Accreditation Board for the past five years.
Keith’s areas of expertise include:
- Microbiological assays for food, medical device, and environmental test matrixes.
- Environmental chemistry of water and wastewater.
- Biocompatibility testing of medical devices.
- ISO/IEC 17025:2017
- AOAC International – guidelines for food laboratories program requirements
- 21 CFR Part 58, GLP program requirements
- EPA NLLAP program requirements
- AAFCO program requirements
- FDA ASCA Pilot program for Biocompatibility
- Michigan Cannabis Regulatory Agency program requirements
- ISO 20387 Biobanking