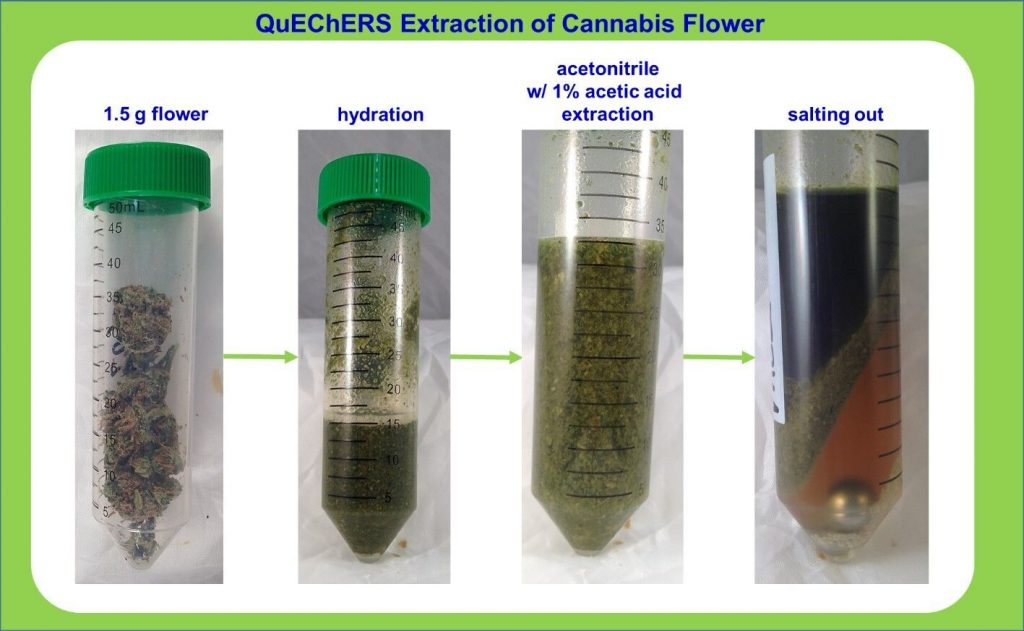
Determination of Toxic Metals in Cannabis
Heavy metals are common environmental contaminants often resulting from mining operations, industrial waste, automotive emissions, coal fired power plants, amount other sources. Several remediation strategies exist that are common for the reduction/elimination of metals in the environment. Phytoremediation is one method for removing metals from soil, utilizing plants to uptake metals which then bioaccumulate in the plant matter. In one study, cesium concentrations were found to be 8,000 times greater in the plant roots compared to the surrounding water in the soil. In 1998, cannabis was specifically tested at the Chernobyl nuclear disaster site for its ability to remediate the contaminated soil. These examples demonstrate that cannabis must be carefully cultivated to avoid the uptake of toxic metals. Possible sources would not only include the growing environment, but also materials such as fertilizers. Many states publish metal content in fertilizer products allowing growers to select the cleanest product for their plants. For cannabis plant material and concentrates several states have specific limits for cadmium (Cd), Lead (Pb), Arsenic (As) and Mercury (Hg), based on absolute limits in product or daily dosage by body weight.
Analytical Approaches to Metals Determination

Flame Atomic Absorption Spectroscopy (Flame AA) and Graphite Furnace Atomic Absorption Spectroscopy (GFAA) are both techniques that determine both the identity and quantity of specific elements. For both of these techniques, the absorption in intensity of a specific light source is measured following the atomization of the sample digestate using either a flame or an electrically heated graphite tube. Reference standards are analyzed prior to the samples in order to develop a calibration that relates the concentration of each element relative to its absorbance. For these two techniques, each element is often determined individually, and the light source, most commonly a hollow cathode lamp (HLC) or electrodeless discharge lamp (EDL) are specific for each element. The two most common types of Atomic Emission Spectroscopy (AES) are; Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES) and ICP-Mass Spectrometry (ICP-MS). Both of these techniques use an argon plasma for atomization of the sample digestates. This argon plasma is maintained using a radio frequency generator that is capable of atomization and excitation of the majority of the elements on the periodic table. Due to the considerably higher energy of the plasma-based instruments, they are more capable than the flame or furnace based systems for measurement of a wide range of elements. Additionally, they are based on optical emission, or mass spectrometric detection, and are capable of analysis of all elements at essentially the same time.
Technique Selection
Flame AA is easy to use, inexpensive and can provide reasonable throughput for a limited number of elements. However, changes to light sources and optical method parameters are necessary when determining different metals. GFAA is also limited by similar needs to change the light sources, though it is capable of greater sensitivity for most elements as compared to flame AA. Runtimes are on the order of three minutes per element for each sample, which can result in lower laboratory throughput and greater sample digestate consumption. While the sensitivity of the absorption techniques is reasonable, the dynamic range can be more limited requiring re-analyses and dilutions to get the sample within the calibration range. ICP-OES allows the simultaneous analysis of over 70 elements in approximately a minute per sample with a much greater linear dynamic range. ICP-OES instruments cost about 2-5 times more than AA instruments. ICP-MS generally has the greatest sensitivity (sub-parts-per-trillion, for some elements) with the ability to determine over 70 elements per minute. Operator complexity, instrument expense and MS stability, as well as cost are some of the disadvantages. The US FDA has a single laboratory validated method for ICP-MS for elements in food using microwave assisted digestion, and New York State recently released a method for the analysis of metals in medical cannabis products by ICP-MS (NYS DOH LINC-250).
The use of fertilizers, and other materials, with low metal content is one step necessary to providing a safe product and maintaining customer confidence. The state-by-state cannabis regulations will continue to evolve which will require instrumentation that is flexible enough to quickly accommodate added metals to the regulatory lists, lower detection limits while adding a high level of confidence in the data.
























 So what happens if a cannabis lab uses non-certified reference materials? Labs might save money in the short term. CRMs are slightly more expensive than a non-certified reference material, but will increase the defensibility of a lab’s data. Using a reference material created in-house or from a non-accredited vendor can lead to less-than-accurate results. A non-certified reference material has a greater chance of being made incorrectly. The publication of incorrect data damages the credibility of the testing lab and could lead to legal action against the lab from damaged parties.
So what happens if a cannabis lab uses non-certified reference materials? Labs might save money in the short term. CRMs are slightly more expensive than a non-certified reference material, but will increase the defensibility of a lab’s data. Using a reference material created in-house or from a non-accredited vendor can lead to less-than-accurate results. A non-certified reference material has a greater chance of being made incorrectly. The publication of incorrect data damages the credibility of the testing lab and could lead to legal action against the lab from damaged parties.