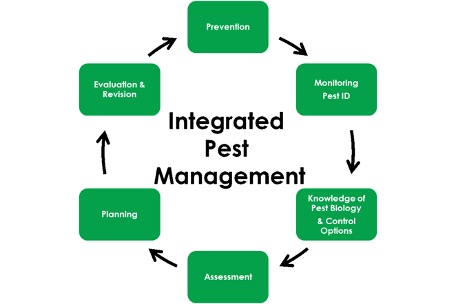
This is the sixth and final in the series of articles designed to introduce an integrated pest management framework for cannabis cultivation facilities. To see Part One, an overview of the plan and pest identification, click here. For Part Two, on pest monitoring and record keeping, click here. For Part Three, on preventative measures, click here. For Part Four, control methods, click here. For Part Five, pest control action thresholds, click here.
This is Part 6: Emergency Response
When all prevention efforts have failed and your escalation procedures must be implemented, your emergency response document takes the stage.

It sounds obvious, but your emergency response document is your team’s guide to structure your response to an emergency. This begins with the simple definition of what is an emergency for your business. Emergencies can be to your personnel (personal injury) or your infrastructure (broken pipes/floods, power failure), and finally, a pest or pathogen outbreak that threatens the entire facility (insects/fungus, molds). Be sure to get the advice of your local service providers on the important things to put in to your response plan. This article is far from an exhaustive list, but it can get you started quickly with the basics for example purposes.
Personal Injury
Personal injuries are the events where you would call your local fire or police resources after stabilizing trauma events. Examples are chemical exposure, cuts, lacerations or broken bones from falls or crush events, burns, electric shock or earthquake or weather events. Injury response is to assess, call for medical assistance if appropriate, provide first aid and stabilize the injured, move to safety if possible, treat the injury and after the event is over and still fresh in everyone’s mind, consider what can be done to avoid the repeat of this or similar events in the future. Work those changes into your standard operating procedures.
Emergency Response to Facility Events

Whether the event is broken pipes or flooding, power failure or interruption, fire, HVAC failure or weather event, emergencies come in all sizes possible. It is likely that you built up a plan for emergency response as part of your city permitting process. Be sure to use those experts to refine your plan to include your operations.
Broken pipes start with the basics of turning off the source feeds and fixing the plumbing. If the water is actually rich fertilizer nutrients, cleaning and disinfectant is necessary as part of the drying and mop up process.
Environmental damage from fire, HVAC or weather event, lead to immediate treatment to try and save the current crops. This would include manual watering/misting, portable heater/cooler/CO2 burners. Verifying that backup power supplies turned on as planned. Are emergency fixes sufficient to power or run the systems necessary for plant life until power is returned?
Cultivation Events

This entire paper has been about pest management, so emergency is expected to mean a pest or pathogen outbreak. We defined the escalated response actions up to the point of direct action and chemical interventions in chapters four and five. Your emergency response plan takes those actions to a site wide effort. Identify the pest and location/s that are causing the crisis, isolate the infested plants, remove the infected materials, clean, disinfect, and purify the contacted surfaces. Follow your plan and contact your emergency leaders.
Emergency Response Team
Your emergency response document identifies each of your team leaders and executives that are to be contacted in the event of an emergency. These leaders should be identified in the document with contact details and methods/on-call schedules for days and times of responsibility (after normal hours and holidays included). Someone is always on-call. The personal injury, facility and cultivation lead responsible should be identified and aware that they are the assigned resource and to treat emergencies as a priority.

In Conclusion
We have covered an example integrated pest management philosophy from prevention through observation to limiting expansion to treatment and review. This continuous monitoring and learning process is a living document of standard operating procedures for any facility.
The attention of your team, their scouting observations, and attention to detail give you an opportunity to address and restrict any pest outbreak before it destroys your crop. Teach your operators well and reward them for their attention to your plan.
Clean and sterilize your facilities regularly. Preventing the emergence of pests will pay for the investment in a multitude of ways in both savings and profits. Plan your response thresholds and use traps to monitor your escalating protections. Target your treatments and remediations to match the threats to your harvests. As a last resort, apply approved chemical treatments judiciously to minimize the impact on non-target organisms.
Evaluate the effectiveness of your plan on an annual basis. Put your improvements to work for you to minimize your pest footprint and to increase your profits in every harvest.
For a copy of the complete Integrated Pest Management guide, download the document here.