Cannabis extraction has been used as a broad term for what can best be described as cannabis processing. A well-thought-out cannabis process goes far beyond just extraction, largely overlapping with cultivation on the front-end and product development on the back-end1. With this in mind, four pillars emerge as crucial capabilities for developing a cannabis process: Cultivation, Extraction, Analytics and Biochemistry.
The purpose and value of each pillar on their own is clear, but it is only when combined that each pillar can be optimized to provide their full capacities in a well-designed process. As such, it is best to define the goals of each pillar alone, and then explain how they synergize with each other.
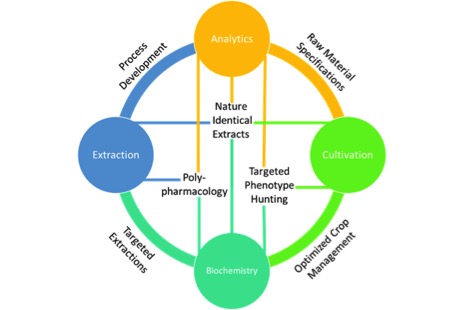
At the intersection of each pillar, specific technology platforms exist that can effectively drive an innovation and discovery cycle towards the development of ideal products.Cultivation is the foundation of any horticultural process, including cannabis production. Whether the goal be to convert pigments, flavors or bioactive compounds into a usable form, a natural process should only utilize what is provided by the raw material, in this case cannabis flower. That means cultivation offers a molecular feedstock for our process, and depending on our end goals there are many requirements we may consider. These requirements start as simply as mass yield. Various metrics that can be used here include mass yield per square foot or per light. Taken further, this yield may be expressed based not only on mass, but the cannabinoid content of the plants grown. This could give rise to a metric like CBD or THC yield per square foot and may be more representative of a successful grow. Furthermore, as scientists work to learn more about how individual cannabinoids and their combinations interact with the human body, cultivators will prioritize identifying cultivars that provide unique ratios of cannabinoids and other bioactive compounds consistently. Research into the synergistic effect of terpenes with cannabinoids suggests that terpene content should be another goal of cultivation2. Finally, and most importantly, it is crucial that cultivation provide clean and safe materials downstream. This means cannabis flower free of pesticides, microbial growth, heavy metals and other contaminants.
Extraction is best described as the conversion of target molecules in cannabis raw material to a usable form. Which molecules those are depends on the goals of your product. This ranges from an extract containing only a pure, isolated cannabinoid like CBD, to an extract containing more than 100 cannabinoids and terpenes in a predictable ratio. There are countless approaches to take in terms of equipment and process optimization in this space so it is paramount to identify which is the best fit for the end-product1. While each extraction process has unique pros and cons, the tunability of supercritical carbon dioxide provides a flexibility in extraction capabilities unlike any other method. This allows the operator to use a single extractor to create extracts that meet the needs of various product applications.
Analytics provide a feedback loop at every stage of cannabis production. Analytics may include gas chromatography methods for terpene content3 or liquid chromatography methods for cannabinoids 3, 4, 5. Analytical methods should be specific, precise and accurate. In an ideal world, they can identify the compounds and their concentrations in a cannabis product. Analytics are a pillar of their own due simply to the efforts required to ensure the quality and reliability of results provided as well as ongoing optimization of methods to provide more sensitive and useful results. That said, analytics are only truly harnessed when paired with the other three pillars.
Biochemistry can be split into two primary focuses. Plant biochemistry focuses back towards cultivation and enables a cannabis scientist to understand the complicated pathways that give rise to unique ratios of bioactive molecules in the plant. Human biochemistry centers on how those bioactive molecules interact with the human endocannabinoid system, as well as how different routes of administration may affect the pharmacokinetic delivery of those active molecules.
Each of the pillars require technical expertise and resources to build, but once established they can be a source of constant innovation. Fig. 1 above shows how each of these pillars are connected. At the intersection of each pillar, specific technology platforms exist that can effectively drive an innovation and discovery cycle towards the development of ideal products.
For example, at the intersection of analytics and cultivation I can develop raw material specifications. This sorely needed quality measure could ensure consistencies in things like cannabinoid content and terpene profiles, more critically they can ensure that the raw material to be processed is free of contamination. Additionally, analytics can provide feedback as I adjust variables in my extraction process resulting in optimized methods. Without analytics I am forced to use very rudimentary methods, such as mass yield, to monitor my process. Mass alone tells me how much crude oil is extracted, but says nothing about the purity or efficiency of my extraction process. By applying plant biochemistry to my cultivation through the use of analytics I could start hunting for specific phenotypes within cultivars that provide elevated levels of specific cannabinoids like CBC or THCV. Taken further, technologies like tissue culturing could rapidly iterate this hunting process6. Certainly, one of the most compelling aspects of cannabinoid therapeutics is the ability to harness the unique polypharmacology of various cannabis cultivars where multiple bioactive compounds are acting on multiple targets7. To eschew the more traditional “silver bullet” pharmaceutical approach a firm understanding of both human and plant biochemistry tied directly to well characterized and consistently processed extracts is required. When all of these pillars are joined effectively we can fully characterize our unique cannabis raw material with targeted cannabinoid and terpene ratios, optimize an extraction process to ensure no loss of desirable bioactive compounds, compare our extracted product back to its source and ensure we are delivering a safe, consistent, “nature identical” extract to use in products with predictable efficacies.
Using these tools, we can confidently set about the task of processing safe, reliable and well characterized cannabis extracts for the development of world class products.
[1] Sweeney, C. “Goal-Oriented Extraction Processes.” Cannabis Science and Technology, vol 1, 2018, pp 54-57.
[2] Russo, E. B. “Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects.” British Journal of Pharmacology, vol. 163, no. 7, 2011, pp. 1344–1364.
[3] Giese, Matthew W., et al. “Method for the Analysis of Cannabinoids and Terpenes in Cannabis.” Journal of AOAC International, vol. 98, no. 6, 2015, pp. 1503–1522.
[4] Gul W., et al. “Determination of 11 Cannabinoids in Biomass and Extracts of Different Varieties of Cannabis Using high-Performance Liquid Chromatography.” Journal of AOAC International, vol. 98, 2015, pp. 1523-1528.
[5] Mudge, E. M., et al. “Leaner and Greener Analysis of Cannabinoids.” Analytical and Bioanalytical Chemistry, vol. 409, 2017, pp. 3153-3163.
[6] Biros, A. G., Jones, H. “Applications for Tissue Culture in Cannabis Growing: Part 1.” Cannabis Industry Journal, 13 Apr. 2017, www.cannabisindustryjournal.com/feature_article/applications-for-tissue-culture-in-cannabis-growing-part-1/.
[7] Brodie, James S., et al. “Polypharmacology Shakes Hands with Complex Aetiopathology.” Trends in Pharmacological Sciences, vol. 36, no. 12, 2015, pp. 802–821.



