With the state led legalization of both adult recreational and medical cannabis, there is a need for comprehensive and reliable analytical testing to ensure consumer safety and drug potency. Cannabis-testing laboratories receive high volumes of test requests from cannabis cultivators for testing quantitative and qualitative aspects of the plant. The testing market is growing as more states bring in stricter enforcement policies on testing. As the number of testing labs grow, it is anticipated that the laboratories that are now servicing other markets, including high throughput contract labs, will cross into cannabis testing as regulations free up. As the volume of tests each lab performs increases, the need for laboratories to make effective use of time and resource management, such as ensuring accurate and quick results, reports, regulatory compliance, quality assurance and many other aspects of data management becomes vital in staying competitive.
Cannabis Testing Workflows
To be commercially competitive, testing labs offer a comprehensive range of testing services. These services are available for both the medical and recreational cannabis markets, including:
- Detection and quantification of both acid and neutral forms of cannabinoids
- Screening for pesticide levels
- Monitoring water activity to indicate the possibility of microbiological contamination
- Moisture content measurements
- Terpene profiling
- Residual solvents and heavy metal testing
- Fungi, molds, mycotoxin testing and many more
Although the testing workflows differ for each test, here is a basic overview of the operations carried out in a cannabis-testing lab:
- Cannabis samples are received.
- The samples are processed using techniques such as grinding and homogenization. This may be followed by extraction, filtration and evaporation.
- A few samples will be isolated and concentrated by dissolving in solvents, while others may be derivatized using HPLC or GC reagents
- The processed samples are then subjected to chromatographic separation using techniques such as HPLC, UHPLC, GC and GC-MS.
- The separated components are then analyzed and identified for qualitative and quantitative analysis based on specialized standards and certified reference materials.
- The quantified analytical data will be exported from the instruments and compiled with the corresponding sample data.
- The test results are organized and reviewed by the lab personnel.
- The finalized test results are reported in a compliant format and released to the client.
In order to ensure that cannabis testing laboratories function reliably, they are obliged to follow and execute certain organizational and regulatory protocols throughout the testing process. These involve critical factors that determine the accuracy of testing services of a laboratory.
Factors Critical to a Cannabis Testing Laboratory
- Accreditations & Regulatory Compliance: Cannabis testing laboratories are subject to regulatory compliance requirements, accreditation standards, laboratory practices and policies at the state level. A standard that most cannabis testing labs comply to is ISO 17025, which sets the requirements of quality standards in testing laboratories. Accreditation to this standard represents the determination of competence by an independent third party referred to as the “Accreditation Body”. Accreditation ensures that laboratories are adhering to their methods. These testing facilities have mandatory participation in proficiency tests regularly in order to maintain accreditation.
- Quality Assurance, Standards & Proficiency Testing: Quality assurance is in part achieved by implementing standard test methods that have been thoroughly validated. When standard methods are not available, the laboratory must validate their own methods. In addition to using valid and appropriate methods, accredited laboratories are also required to participate in appropriate and commercially available Proficiency Test Program or Inter-Laboratory Comparison Study. Both PT and ILC Programs provide laboratories with some measure of their analytic performance and compare that performance with other participating laboratories.
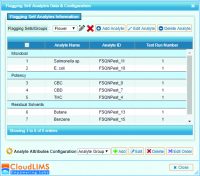
CloudLIMS Cannabis Testing LIMS: Multi-analyte Configuration for Cannabis Testing Services - Real-time Collaboration: Testing facilities generate metadata such as data derived from cannabis samples and infused products. The testing status and test results are best served for compliance and accessibility when integrated and stored on a centralized platform. This helps in timely data sharing and facilitates informed decision making, effective cooperation and relationships between cannabis testing facilities and growers. This platform is imperative for laboratories that have grown to high volume throughput where opportunities for errors exist. By matching test results to samples, this platform ensures consistent sample tracking and traceability. Finally, the platform is designed to provide immediate, real-time reporting to individual state or other regulatory bodies.
- Personnel Management: Skilled scientific staff in cannabis-testing laboratories are required to oversee testing activities. Staff should have experience in analytical chromatography instruments such as HPLC and GC-MS. Since samples are often used for multi-analytes such as terpenes, cannabinoids, pesticides etc., the process often involves transferring samples and tests from one person to another within the testing facility. A chain of custody (CoC) is required to ensure traceability and ‘ownership’ for each person involved in the workflow.
LIMS for Laboratory Automation
Gathering, organizing and controlling laboratory-testing data can be time-consuming, labor-intensive and challenging for cannabis testing laboratories. Using spreadsheets and paper methods for this purpose is error-prone, makes data retrieval difficult and does not allow laboratories to easily adhere to regulatory guidelines. Manual systems are cumbersome, costly and lack efficiency. One way to meet this challenge is to switch to automated solutions that eliminate many of the mundane tasks that utilize valuable human resources.. Laboratory automation transforms the data management processes and as a result, improves the quality of services and provides faster turnaround time with significant cost savings. Automating the data management protocol will improve the quality of accountability, improve technical efficiency, and improve fiscal resources.
A Laboratory Information Management System (LIMS) is a software tool for testing labs that aids efficient data management. A LIMS organizes, manages and communicates all laboratory test data and related information, such as sample and associated metadata, tests, Standard Operating Procedures (SOPs), test reports, and invoices. It also enables fully automated data exchange between instruments such as HPLCs, GC-FIDs, etc. to one consolidated location, thereby reducing transcription errors.
How LIMS Helps Cannabis Testing Labs
LIMS are much more capable than spreadsheets and paper-based tools for streamlining the analytical and operational lab activities and enhances the productivity and quality by eliminating manual data entry. Cloud-enabled LIMS systems such as CloudLIMS are often low in the total cost of acquisition, do not require IT staff and are scalable to help meet the ever changing business and regulatory compliance needs. Some of the key benefits of LIMS for automating a cannabis-testing laboratory are illustrated below [Table 1]:
| Key Functionality | Benefit |
| Barcode label designing and printing | Enables proper labelling of samples and inventory
Follows GLP guidelines |
| Instant data capture by scanning barcodes | Facilitates quick client registration and sample access |
| 3600 data traceability | Saves time and resources for locating samples and other records |
| Inventory and order management | Supports proactive planning/budgeting and real time accuracy |
| Custodian management | Promotes overall laboratory organization by assigning custodians for samples and tests
Maintains the Chain-of-custody (CoC) |
| Test management | Accommodates pre-loaded test protocols to quickly assign tests for incoming samples |
| Accounting for sample and inventory quantity | Automatically deducts sample and inventory quantities when consumed in tests |
| Package & shipment management | Manages incoming samples and samples that have been subcontracted to other laboratories |
| Electronic data import | Electronically imports test results and metadata from integrated instruments
Eliminates manual typographical errors |
| Report management | Generates accurate, customizable, meaningful and test reports for clients
Allows user to include signatures and additional sections for professional use |
| 21 CFR Part 11 compliant | Authenticates laboratory activities with electronic signatures |
| ISO 17025 accreditation | Provides traceable documentary evidence required to achieve ISO 17025 accreditation |
| Audit trail capabilities | Adheres to regulatory standards by recording comprehensive audit logs for laboratory activities along with the date and time stamp |
| Centralized data management | Stores all the data in a single, secure database facilitating quick data retrieval |
| Workflow management | Promotes better data management and resource allocation |
| High-configurability | Enables modification of screens using graphical configuration tools to mirror testing workflows |
| State compliance systems | Integrates with state-required compliance reporting systems and communicates using API |
| Adheres to regulatory compliance | Creates Certificates of Analysis (CoA) to prove regulatory compliance for each batch as well as batch-by-batch variance analysis and other reports as needed. |
| Data security & confidentiality | Masks sensitive data from unauthorized user access
Cloud-based LIMS encrypts data at rest and in-transit while transmission between the client and the server |
| Global accessibility | Cloud-based LIMS provides real-time access to laboratory data from anytime anywhere |
| Real-time collaboration | Cloud-based LIMS enhances real-time communication within a laboratory, between a laboratory and its clients, and across a global organization with multiple sites |
Table 1. Key functionality and benefits of LIMS for cannabis testing laboratories
Upon mapping the present day challenges faced by cannabis testing laboratories, adopting laboratory automation solutions becomes imperative. Cloud-based LIMS becomes a valuable tool for laboratory data management in cannabis testing laboratories. In addition to reducing manual workloads, and efficient resource management, it helps labs focus on productive lab operations while achieving compliance and regulatory goals with ease.
For more information on this, check out a webinar here: Webinar: How to Meet Cannabis Testing Standards and Regulatory Requirements with LIMS by Stephen Goldman, laboratory director at the State of Colorado certified Cannabis testing facility, PhytaTech.