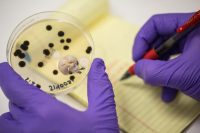
Three health alerts were issued Thursday, November 4th for contaminated cannabis sold to consumers at North Bend, Salem and Eugene dispensaries. Green-Way Medicinal in Salem and Stonies in North Bend sold two strains of cannabis flower found to have high levels of piperonyl butoxide, an ingredient commonly found in pesticides that acts as a synergist to amplify the effects of certain compounds.
The two batches in question, including the strains Pleeze (batch number G6J0039-02) and Dryzl (batch number G6J0039-01), were found to contain the potentially dangerous chemical at levels of 15.39 ppm and 16.24 ppm, respectively. The Oregon Health Authority (OHA) action level for piperonyl butoxide is 2.0 ppm. To see the full health alert, click here.
The dispensary in Eugene, Flowr of Lyfe, sold one strain of cannabis that had levels of the insecticide spinosad over the 0.2-ppm action level. The very popular indica hybrid, Dutch Treat (batch number G6J0018-01), was found to contain 0.9-ppm of spinosad. Though it still tested above the 0.2-ppm action level for that insecticide, it pales in comparison to October’s health alert, where a batch of cannabis had over 200 times the acceptable level of that insecticide. Both spinosad and piperonyl butoxide are considered toxic to humans.
According to the health alert, “All tests were performed by an OHA-accredited and Oregon Liquor Control Commission-licensed laboratory.” It is unclear exactly how or why the cannabis was able to get transported and transferred from the grower to the dispensary and then sold to consumers after failing the pesticide test. According to Jonathan Modie, spokesman for the OHA, they are currently investigating the matter and following up with the dispensaries and growers to find out what happened. “We need to find out how this got transferred in the first place and then sold,” says Modie. “They had access to the test results and should have been able to determine for themselves that these products should not have been sold or transferred.”
“We don’t know, we are still gathering information, there is a risk of civil penalty as well as losing your registration for a dispensary or grower that illegally transferred products that have tested for analytes above the action levels,” says Modie, when asked if punitive measures would be taken. While there are no particular regulations for this scenario in performing a mandatory recall, the OHA is obligated under law to issue health alerts when there is a situation that might affect public health, according to Modie.
“We deal with this with infectious disease outbreaks or during a food borne illness outbreak; if they [the public] can avoid it by hearing from us then we want to get the word out and this is a very similar situation.” For medical patients that purchase potentially contaminated cannabis such as this, it is easy to contact them to have the patient dispose or return the cannabis. Dispensaries are not required to collect information from recreational customers, and most dispensaries do not, which is a major problem when this situation happens, as it has twice in the past two weeks.
“We can never do too much communication,” says Modie. “We will let the public know in any way possible that they should return this product or dispose of it responsibly.”


























 According to Tyler Dautrich, co-founder of Greenhouse Ventures, Lindy Snider is an extraordinarily valuable asset. “Lindy has been essential in the early success that Greenhouse Ventures has experienced to date and we are fortunate to name such an active and respected member of the investment community as our lead advisor,” says Dautrich. The company will be hosting two ten-week semesters in February and September every year. Applicants that are accepted into the program typically receive an average of $60,000 in professional services in exchange for a minor equity stake in their venture. Those accepted applicants are not required to relocate, as virtual participation is available.
According to Tyler Dautrich, co-founder of Greenhouse Ventures, Lindy Snider is an extraordinarily valuable asset. “Lindy has been essential in the early success that Greenhouse Ventures has experienced to date and we are fortunate to name such an active and respected member of the investment community as our lead advisor,” says Dautrich. The company will be hosting two ten-week semesters in February and September every year. Applicants that are accepted into the program typically receive an average of $60,000 in professional services in exchange for a minor equity stake in their venture. Those accepted applicants are not required to relocate, as virtual participation is available.



