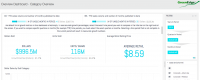
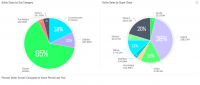
Oregon cannabis regulators began enforcing new rules over the weekend when the October 1st compliance deadline passed. Compared to the relatively cut-and-dried new Colorado regulations, the Oregon cannabis market faces more complex and changing regulatory compliance issues.
The new rules in Oregon address changes to testing, packaging and labeling regulations along with concentration and serving size limits, according to a bulletin published by the Oregon Health Authority (OHA) and the Oregon Medical Marijuana Program (OMMP) earlier this week. Most of the new rules are meant to add safeguards for public health and consumer safety, while putting an emphasis on keeping cannabis away from children.
Around the same time, the Oregon Liquor Control Commission (OLCC) published a bulletin with a new temporary rule that is meant to prevent marketing to children. The OLCC’s temporary rule clarifies “restrictions on product wording commonly associated with products marketed by or to children.” The OLCC reviewed around 500 strain names and found roughly 20 of them that could appeal to children. The OLCC will not approve labels that include strain names like Girl Scout Cookies, Candyland and Charlotte’s Web, among others. This means that breeders and growers have to change strain names on labels like Death Star, Skywalker and Jedi Kush because they contain a reference to the Star Wars franchise, which is marketed to children.

The new testing regulations establish requirements for testing cannabis products for THC and CBD concentrations, water activity, moisture content, pesticides and solvents in concentrates. They also stipulate that ORELAP-accredited laboratories must perform the testing. In the time leading up to the compliance deadline, many lacked confidence that ORELAP would accredit enough laboratories to meet the demand for testing. “We have heard from existing accredited labs that they can meet demand for cannabis product testing,” says Jonathan Modie, spokesman for the OHA. “We don’t yet know how much product requires testing, so we can’t speculate on whether labs will indeed be able to meet demand.” It is still unclear at this time if there are enough laboratories to perform all of the testing for cannabis products in the state.

At this time, 16 laboratories have been accredited for some form of testing, but only four labs have been accredited for pesticide testing. A list of the labs that ORELAP has accredited can be found here. Notably, only one lab is accredited for testing microbiological contaminants, such as E. coli. Testing for microbiological contaminants is not required for all cannabis products sold, rather it is only required upon written request by the OHA or OLCC.
The new labeling and packaging requirements concern testing, consumer education, childproofing and preventing marketing to minors. All cannabis products must contain a label that has been pre-approved by the OLCC. “Cannabis products have to be clearly labeled, showing that is has been tested, or if it has not been tested then it must display ‘does not meet new testing requirements’,” says Modie. “It [the label] must be clear, legible and readable, so they [the consumer] know exactly what it contains, including what cannabis product is inside the package, how much of it, how much THC, and where the product came from.”
According to Modie, it is particularly important that the packaging is not attractive to minors. Cartoons, designs and names that resemble non-cannabis products intended for, or marketed to children, should not be on the packaging or label. “Part of our education to the public and recreational cannabis users focuses on keeping these products out of reach of children in the first place, like storing cannabis in a locked area or an area where a child cannot reach or see,” says Modie. “Our goal is always to protect public health.”
















 According to Tyler Dautrich, co-founder of Greenhouse Ventures, Lindy Snider is an extraordinarily valuable asset. “Lindy has been essential in the early success that Greenhouse Ventures has experienced to date and we are fortunate to name such an active and respected member of the investment community as our lead advisor,” says Dautrich. The company will be hosting two ten-week semesters in February and September every year. Applicants that are accepted into the program typically receive an average of $60,000 in professional services in exchange for a minor equity stake in their venture. Those accepted applicants are not required to relocate, as virtual participation is available.
According to Tyler Dautrich, co-founder of Greenhouse Ventures, Lindy Snider is an extraordinarily valuable asset. “Lindy has been essential in the early success that Greenhouse Ventures has experienced to date and we are fortunate to name such an active and respected member of the investment community as our lead advisor,” says Dautrich. The company will be hosting two ten-week semesters in February and September every year. Applicants that are accepted into the program typically receive an average of $60,000 in professional services in exchange for a minor equity stake in their venture. Those accepted applicants are not required to relocate, as virtual participation is available.






 By implementing ISO 17025 accreditation, the laboratory monitors systems and processes central to analyses in an effort to minimize discrepancies and variability in test results. According to Roger Brauninger, biosafety program manager at A2LA, this type of accreditation demonstrates their competence and commitment to rigorous science. “It is encouraging to have testing laboratories taking ownership of the quality of the work performed,” says Brauninger. “Reliable testing will be imperative to insure safety of the products out on the market as this industry continues to expand.” As the first accreditation of its kind in North America, Brauninger hopes this will open the doors for more cannabis laboratories to acknowledge their role in demonstrating scientific competency for the industry.
By implementing ISO 17025 accreditation, the laboratory monitors systems and processes central to analyses in an effort to minimize discrepancies and variability in test results. According to Roger Brauninger, biosafety program manager at A2LA, this type of accreditation demonstrates their competence and commitment to rigorous science. “It is encouraging to have testing laboratories taking ownership of the quality of the work performed,” says Brauninger. “Reliable testing will be imperative to insure safety of the products out on the market as this industry continues to expand.” As the first accreditation of its kind in North America, Brauninger hopes this will open the doors for more cannabis laboratories to acknowledge their role in demonstrating scientific competency for the industry.