Government regulations keep millions of Americans safe every year by controlling what companies can put in their products and the standards those products must meet to be sold to consumers.
Enter the strange case of legal cannabis: In order for cannabis to be legally distributed by licensed medical professionals and businesses, it must be tested. But unlike other consumable goods, cannabis is not regulated by the FDA. Without an overarching federal policy requiring cannabis testing laboratory accreditation, the testing and laboratory requirements differ greatly across state lines.For medical cannabis specifically, accredited testing facilities are especially important.
To be federally regulated, cannabis would first have to be federally legalized. It turns out that states and businesses alike are not willing to wait for a federal mandate. Many states have begun to adopt standards for cannabis testing and some, such as Ohio, have even announced mandatory ISO/IEC 17025 accreditation for all cannabis testing laboratories. As the industry evolves, increased compliance expectations are certain to evolve in tandem.
Some cannabis labs have even taken the initiative to seek ISO/IEC 17025 accreditation of their own volition. Seth Wong, President of TEQ Analytics Laboratories, shared in a press release:
“By achieving ISO/IEC 17025 accreditation, TEQ Analytical Labs believes that we can address the concerns throughout the cannabis industry regarding insufficient and unreliable scientific analysis by providing our clients with State required tests that are accredited by an international standard.”
Other laboratories, such as DB Labs in Las Vegas and EVIO Labs in Florida are also leading the accreditation charge in their respective states, ahead of any state mandates.
There are key reasons why accreditation in cannabis testing labs is important. First and foremost, cannabis is a consumable product. Like fruits and vegetables, cannabis is prone to pesticides, fungi and contaminants. The result of putting a potentially hazardous material on the market without proper and documented testing could lead to a public health crisis. An accredited testing lab, however, will ensure that the cannabis products they test are free from harmful contaminants.

For medical cannabis specifically, accredited testing facilities are especially important. Because many consumers of medical cannabis are immuno-compromised (such as in the case of chemotherapy patients), ensuring that products are free from any and all contaminants is critical. Further, in order to accurately determine both short- and long-term effects of prescribed cannabis consumption, accredited and compliant laboratories are necessary.
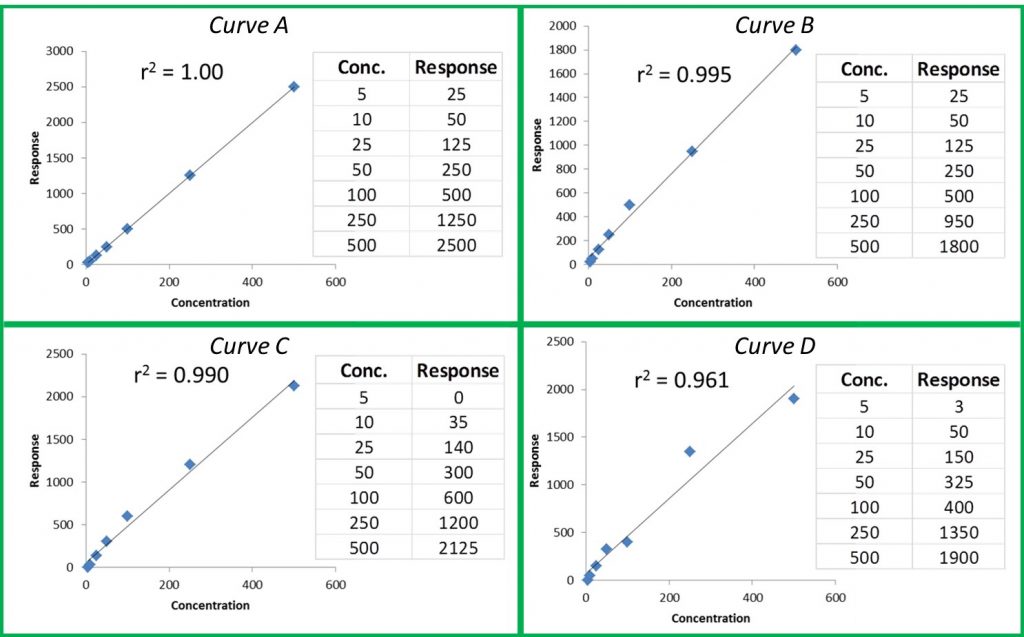
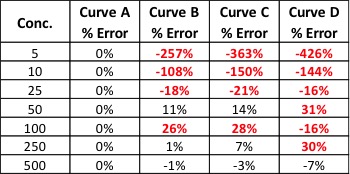
Accreditation standards like ISO/IEC 17025 also provide confidence that testing is performed properly and to an internationally accepted standard. Rather than returning a “pass/fail” rating on products, the Cannabis Safety Institute reports that an ISO/IEC 17025 laboratory is required to produce numerical accuracy percentages in testing for “at a minimum, cannabinoids, pesticides, microbiology, residual solvents, and water activity.” Reliable data sets that can be reviewed by both accreditors and the public foster trust between producers and consumers.
Finally, ISO/IEC 17025 accreditation demonstrates that a laboratory is properly staffed and trained. The Cannabis Safety Institute’s “Standards for Cannabis Testing Laboratories” explains that conducting proper analytical chemistry on cannabinoids (the chemical compounds extracted from cannabis that alter the brain’s neurotransmitter release) requires personnel who have met specific academic and training credentials. A system to monitor, manage and demonstrate proficiency is necessary to achieve and maintain accreditation. With electronic systems in place, this management and documentation minimizes risk and also minimizes administrative time tracking and maintaining training records.
Following the proper steps of a standardized process is key to improving and growing the cannabis industry in coming yearsFor cannabis testing labs, utilizing a comprehensive software solution to achieve and maintain compliance to standards such as ISO/IEC 17025 is key. Absent of a software solution, the necessary compliance requirements can become a significant burden to the organization. Paper tracking systems and complex spreadsheets open up organizations to the likelihood of errors and ultimately risk.
Because ISO/IEC 17025 has clearly defined expectations for training, a software solution also streamlines the training process while simultaneously documenting proficiency. By utilizing role-based trainings, organizations can be confident employees are receiving proper onboarding and in-service training. Additionally, the effectiveness of training can be proven with reports, which results in smoother audits and assessments.
Following the proper steps of a standardized process is key to improving and growing the cannabis industry in coming years- which means utilizing technology tools such as electronic workflows to ensure proper process controls. Beyond adding critical visibility, workflows also create efficiencies that can eliminate the need to increase staffing as companies expand and grow.
For an industry that is changing at a rapid pace, ensuring traceability, efficient processes and visibility across organizations is paramount. Using a system that enables automation, process control, document management and documented training procedures is a step in the right direction. With the proper software tools in place, cannabis testing labs can achieve compliance goals, demonstrate reliable and relevant results and most importantly ensure consumer safety.