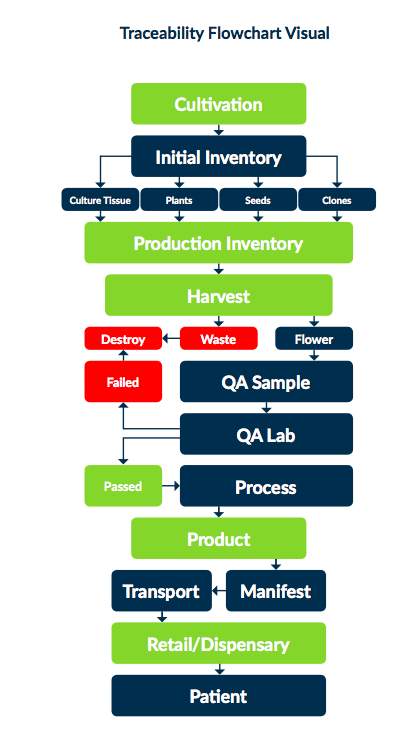
Would you be proud to have your customers and patients tour your production facility? When health inspectors or enforcement personnel arrive at your location is there sense of panic or pride?
When you have detailed systems in place, inspections should be informative, not stressful. Keep in mind that in the cannabis industry, products are often created for patients. Patients may have a compromised immune system and thus are more susceptible to food borne illnesses, pesticides and other contaminants.
Are you and your team doing everything you can to produce a wholesome and safe product?
According to the World Health Organization, Good Manufacturing Process (GMP) “is a system for ensuring that products are consistently produced according to quality standards.”
GMP is the proactive part of quality assurance. It is designed to minimize the risks involved in all steps of the manufacturing process. A basic tenant of GMP is that quality cannot be tested into a product. It must be built into each batch of product during all stages of the manufacturing process.
GMPs involve much more than most people think. A common misconception is that GMP only covers the process of manufacturing itself. GMPs actually cover all aspects of the production process:
- Materials
- Premises
- Equipment
- Storage
- Record Keeping
- Staff Training to Hygiene
- How Complaints Are Handled
GMP & The Cannabis Industry
In most industries, agencies that control licensing for the manufacture and sale of a product recommend GMPs, or guidelines to business owners. These guidelines provide minimum requirements that a manufacturer must meet to assure that products are of high quality and do not pose any risk to the consumer or public. The guidelines generally become the basis of regulation for that industry.
In the United States, the Food and Drug Administration (FDA) recommends guidelines for anything food, drug or pharmaceutical related.
Because cannabis still remains illegal at the federal level, none of the federal agencies that would normally develop good manufacturing guidelines have done so. This has left state lawmakers and business owners on their own to navigate this new and rapidly developing industry.
The Foundation of Cannabis Unified Standards (FOCUS) has developed standards with a mission to protect public health, consumer safety and safeguard the environment by promoting integrity in the cannabis industry.
The comprehensive implementation of cannabis specific good manufacturing practices, like the FOCUS standards, across all aspects of the industry will assist business owners and regulators alike, addressing quality proactively at every step in the process, which is critical to protecting consumer safety and public health – and the overall success of a nascent and divisive industry like cannabis.
The FOCUS standards are completing the final phase of development, a thirty-day public review and comment period before being released for use in the marketplace in June. These voluntary consensus-based standards are built on GMPs drawn from agriculture, food production, chemical management, OTCs, pharmaceuticals, and other relevant industries. In addition, the standards draw best practices from the cannabis industry, as well as those published in OSHA, FDA, FTC, CDC, ISO, code of federal regulations and various state-level cannabis regulations.
There are many aspects of creating and implementing GMPs. Here are three to be aware of:
- Get the facility design right from the start: It’s much easier to be GMP compliant if the design and construction of the facilities and equipment are right from the start. It is important to embody GMP principles and use GMPs to drive every decision.
- Document what you do and do what you document: Having good procedures in place to ensure a controlled and consistent performance is an essential part of GMP. Procedures should be clear, concise, logical, and available to everyone.
- Keep good records: Keeping accurate records is an essential part of GMP. It helps convey that you are following procedures and demonstrates that processes are known and under control. If it’s not written down, it did not happen.
Standards and quality programs in any industry are dynamic by nature. Nothing is static. Standards must constantly be updated to reflect ever-changing market conditions. This is why it is so crucial that regulations are based on them.
To be a standard, there are certain core principals that must be present. However, the goal of a standard is to guide an industry without impeding or controlling it. This is why there is so much inherent value in implementing standards. They bring enough structure to help reduce costs and increase efficiency, but not so much control that individual nuances or creativity is affected.
It is much less expensive to be proactive. Recovering from a recall or contaminated product can not only be costly, it is a massive hit to the company’s reputation. It may take years for sales to recover, and for consumers to trust the product again. Where could you and your team enhance your standards and processes?