I was wrong. And that’s a good thing! Based on all available data, I assumed that evaporating ethanol from a cannabis oil/ethanol solution would result in terpene loss. As it turns out, it doesn’t. There are so many beliefs and assumptions about cannabis: Cannabis cures cancer!1 Smoking cannabis causes cancer!2 Sativas help you sleep; Indicas make you creative!3,4 CBD is not psychoactive!5 But are these ‘facts’ backed by science? Have they been experimentally tested and validated?
I postulated a theory, designed experiments to validate it and evaluated the results. Simply putting “cannabis backed by science” on your label does not solve the problem. Science is not a marketing term. It’s not even a fixed term. The practice of science is multifaceted and sometimes confusing. It evolved from the traditional model of Inductivism, where observations are used in an iterative process to refine a law/theory that can generalize such observations.6 Closely related is Empiricism, which posits that knowledge can only come from observation. Rationalism, on the other hand, believes that certain truths can be directly grasped by one’s intellect.7 In the last century, the definition of science was changed from the method by which we study something, such as Inductivism or Rationalism, and refocused on the way we explain phenomena. It states that a theory should be considered scientific if, and only if, it is falsifiable.8 All that means is that not the way we study something is what makes it scientific, but the way we explain it.
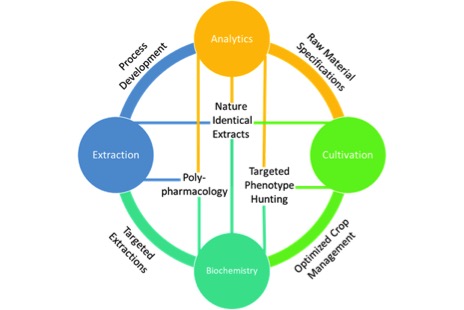
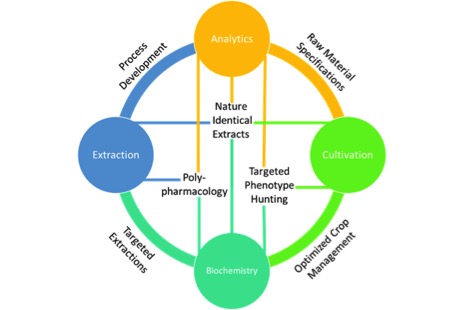
I wonder how can we use empirical observations and rational deliberations to solve the questions surrounding cannabis? And more importantly, how can we form scientific theories that are falsifiable? Cannabis, the plant, the drug, has long been withheld from society by its legal status. As a result, much of what we know, in fact, the entire industry has thrived in the shadows away from rigorous research. It’s time for this to change. I am particularly concerned by the lack of fundamental research in the field. I am not even talking about large questions, like the potential medical benefit of the plant and its constituents. Those are for later. I’m talking about fundamental, mundane questions like how many lumens per square centimetre does the plant need for optimal THC production? What are the kinetics of cannabis extraction in different solvents? What are the thermodynamics of decarboxylation? Where do major cannabinoids differ or align in terms of water solubility and viscosity?
The lack of knowledge and data in the cannabis field puts us in the precarious position of potentially chasing the wrong goals, not to mention wasting enormous amounts of time and money. Here’s a recent example drawn from personal experience:Certainly, I cannot be the only one who has made an incorrect assumption based on anecdotes and incomplete data?
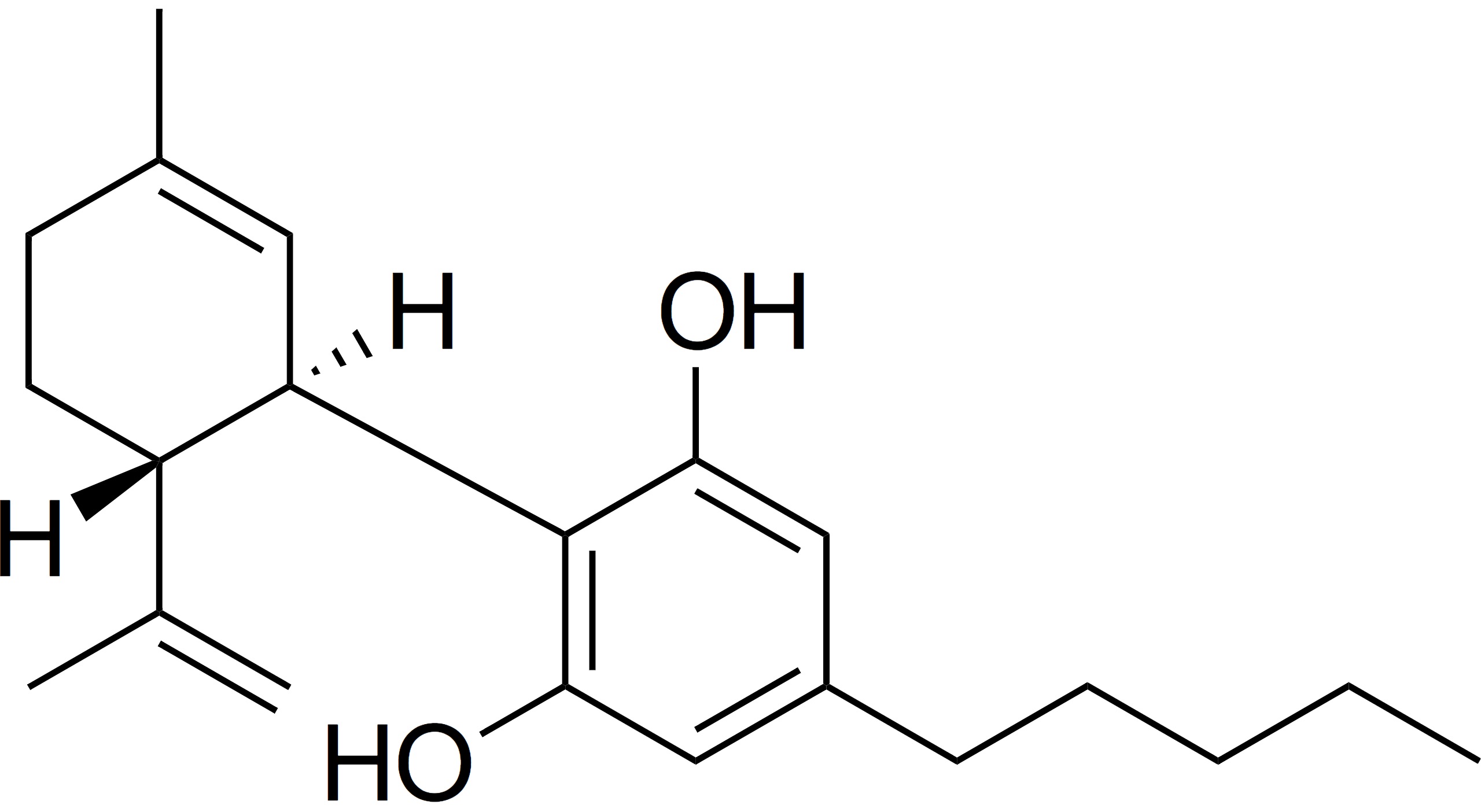
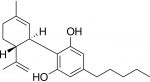
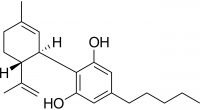
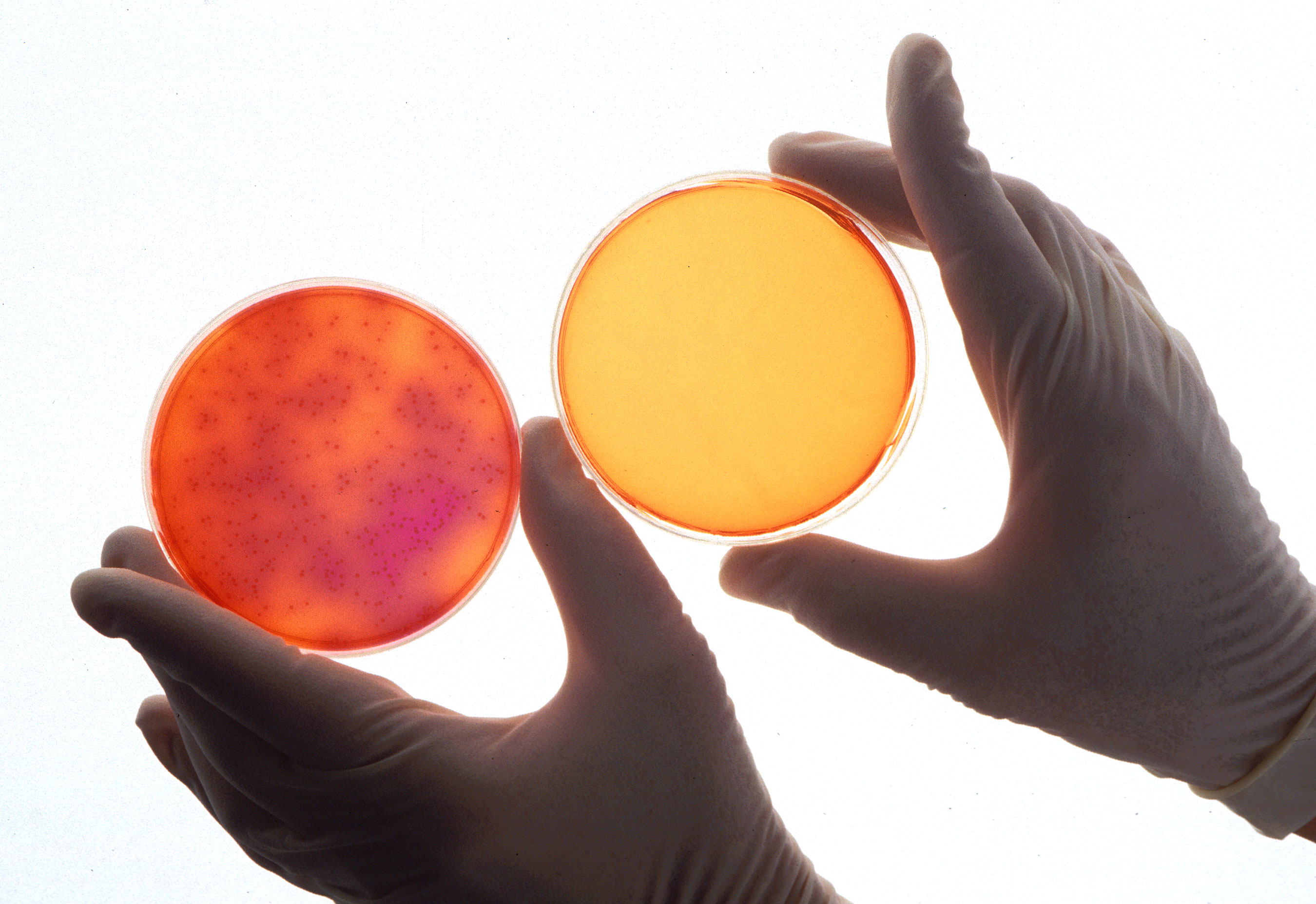
Some of the most common steps in cannabis oil production involve ethanol solutions. Ethanol is commonly removed from extraction material under reduced pressure and elevated heat in a rotary evaporator. I expected that this process would endanger the terpenes in the oil – a key component of product quality. My theory was that volatile terpenes9 would be lost in the rotary evaporator during ethanol10 removal. The close values of vapor pressure for terpenes and ethanol make this a reasonably assumed possibility.11 In the summer of 2018, I finally got the chance to test it. I designed experiments at different temperatures and pressures, neat and in solution, to quantify the terpene lost in ethanol evaporation. I also considered real life conditions and limitations of cannabis oil manufacturers. After all the experiments were done, the results unequivocally showed that terpenes do not evaporate in a rotary evaporator when ethanol is removed from cannabis extracts.12 As it turns out, I was wrong.
We, as an industry, need to start putting money and effort into fundamental cannabis research programs. But, at least I ran the experiments! I postulated a theory, designed experiments to validate it and evaluated the results. At this point, and only this point, can I conclude anything about my hypothesis, even if that is that my working theory needs to be revised. Certainly, I cannot be the only one who has made an incorrect assumption based on anecdotes and incomplete data?
There is a particular danger when using incomplete data to form conclusions. There are many striking examples in the medical literature and even the casual observer might know them. The case of hormone replacement therapy for menopause and the associated risks of cardiovascular diseases showed how observational studies and well-designed clinical trials can lead to contradicting results.13 In the thirties of the last century, lobotomy became a cure-all technique for mental health issues.14 Dr. Moniz even won the Nobel Prize in Medicine for it.15 And it must come as no surprise when WIRED states “that one generation’s Nobel Prize-winning cure is another generation’s worst nightmare.”16 And with today’s knowledge is impossible to consider mercury as a treatment for syphilis, but that is exactly what it was used as for many centuries.17 All those examples, but the last one in particular should “be a good example of the weight of tradition or habit in the medical practice, […] of the necessity and the difficulties to evaluate the treatments without error.”18 There is the danger that we as cannabis professionals fall into the same trap and believe the old stories and become dogmatic about cannabis’ potential.
We, as an industry, need to start putting money and effort into fundamental cannabis research programs. That might be by sponsoring academic research,19 building in-house research divisions,20 or even building research networks.21 I fully believe in the need for fundamental cannabis research, even the non-sexy aspects.22 Therefore, I set up just that: an independent research laboratory, focused on fundamental cannabis research where we can test our assumptions and validate our theories. Although, I alone cannot do it all. I likely will be wrong somewhere (again). So, please join me in this effort. Let’s make sure cannabis science progresses.
References
- No, it does not. There are preliminary in-situ studies that point at anti-cancer effects, but its more complicated. The therapeutic effects of Cannabis and cannabinoids: An update from the National Academies of Sciences, Engineering and Medicine report, Abrams, Donald I., European Journal of Internal Medicine, Volume 49, 7 – 11
- No, it does not. National Academies of Sciences, Engineering, and Medicine. 2017. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press. https://doi.org/10.17226/24625.
- No, it does not. The chemical profile of the plant dictates the biological effects on humans, not the shape of the leaf. Justin T. Fischedick, Cannabis and Cannabinoid Research, Volume: 2 Issue 1: March 1, 2017
- Indica and Sativa are outdated terms. Piomelli D, Russo EB. The Cannabis sativa versus Cannabis indica debate: An Interview with Ethan Russo, MD. Cannabis Cannabinoid Res 2016; 1: 44–46.
- No, it is. CBD’s supposed “calming effects” is indeed a psychoactive effect. However, it is not intoxicating like THC. Russo E.B., Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects.Br. J. Pharmacol. 2011; 163: 1344-1364
- As attributed to Francis Bacon.
- See the work by philosopher Baruch Spinoza.
- As theorized by Karl Popper.
- Monoterpenes have a vapor pressure in the low to mid hundreds of Pascals at room temperature.
- Vapor pressure of 5.95 kPa at 20˚C.
- Furthermore, there is always the possibility of azeotropes in complex mixtures. Azeotropes are mixtures of two or more liquids that have different boiling points individually, but in mixture boil together.
- Terpene Retention via Rotary Evaporator Application Note, Heidolph North America
- https://www.pharmaceutical-journal.com/research/review-article/establishing-the-risk-related-to-hormone-replacement-therapy-and-cardiovascular-disease-in-women/20202066.article?firstPass=false
- https://psychcentral.com/blog/the-surprising-history-of-the-lobotomy/
- https://en.wikipedia.org/wiki/António_Egas_Moniz
- https://www.wired.com/2011/03/lobotomy-history/
- https://www.infezmed.it/media/journal/Vol_21_4_2013_10.pdf
- https://www.ncbi.nlm.nih.gov/pubmed/11625051
- Canopy Growth funds a professorship of cannabis science at UBC. Tilray collaborates with UCSD on a phase I/II clinical trial.
- For examples see: NIBR, PMISCIENCE.
- For examples see: CEMI, theAIRnet, Future Sky.
- Research that does not lead to short-term stock value spikes but long-term progress