Hemp
The hemp industry is the marijuana industry’s half-sister. Both are variations of the plant Cannabis sativa and both were made illegal in 1937 with the passing of The Marijuana Tax Act. Despite this federal status, in recent years 33 individual states have legalized some type of medicinal marijuana use and 11 states now allow legal recreational marijuana within their borders. This prompted congress to modify the legality of hemp which was addressed in The Agricultural Act of 2014, but it only allowed hemp to be used for research purposes. The Agriculture Improvement Act of 2018 (known as the 2018 Farm Bill) that was signed into law on December 20, 2018 was a huge step forward for public access to hemp and hemp products. The 2018 Farm Bill legalized the growing of hemp in states with a state-mandated hemp program and removed hemp and its derivatives from Drug Enforcement Administration (DEA) Schedule I status. Schedule I drugs, substances, or chemicals are defined as drugs with no currently accepted medical use and a high potential for abuse. Some examples of Schedule I drugs are heroin, lysergic acid diethylamide (LSD), 3,4-methylenedioxymethamphetamine (ecstasy), methaqualone, and peyote. Consumers and the cannabis industry alike were very excited about this legalization of hemp…. but that was when the confusion began.
FDA & Hemp
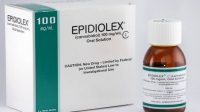
 Within two hours of the 2018 Farm Bill being signed, the Commissioner of the FDA, Dr. Scott Gottlieb, issued a statement reiterating the FDA stance on cannabis products and cannabidiol (CBD) in products for human and animal consumption: “Congress explicitly preserved the agency’s current authority to regulate products containing cannabis or cannabis-derived compounds under the Federal Food, Drug, and Cosmetic Act (FD&C Act) and section 351 of the Public Health Service Act.” Currently the FDA only permits CBD products submitted as an Investigational New Drug (IND) Application as a pharmaceutical. There is only one such accepted CBD product, Epidiolex, manufactured by G.W. Pharma. All other CBD products are illegal for interstate shipment.
Within two hours of the 2018 Farm Bill being signed, the Commissioner of the FDA, Dr. Scott Gottlieb, issued a statement reiterating the FDA stance on cannabis products and cannabidiol (CBD) in products for human and animal consumption: “Congress explicitly preserved the agency’s current authority to regulate products containing cannabis or cannabis-derived compounds under the Federal Food, Drug, and Cosmetic Act (FD&C Act) and section 351 of the Public Health Service Act.” Currently the FDA only permits CBD products submitted as an Investigational New Drug (IND) Application as a pharmaceutical. There is only one such accepted CBD product, Epidiolex, manufactured by G.W. Pharma. All other CBD products are illegal for interstate shipment.
 Every product for sale in the US which is either ingested or applied to a human or animal body has a regulatory category in the FDA. Hemp-derived CBD products will have to fit into one of those categories or it will not be legal. Many hemp manufacturing companies will argue with the illegality of CBD products, but it will get them nowhere. If you manufacture and sell hemp products inside of a state with a state mandated hemp program, you are legal and protected under state laws, but the minute you sell across state lines, it becomes the jurisdiction of the federal government and, more specifically, the FDA. Section 10113 of the 2018 Farm Bill states that (c) Nothing in this subtitle shall affect or modify:
Every product for sale in the US which is either ingested or applied to a human or animal body has a regulatory category in the FDA. Hemp-derived CBD products will have to fit into one of those categories or it will not be legal. Many hemp manufacturing companies will argue with the illegality of CBD products, but it will get them nowhere. If you manufacture and sell hemp products inside of a state with a state mandated hemp program, you are legal and protected under state laws, but the minute you sell across state lines, it becomes the jurisdiction of the federal government and, more specifically, the FDA. Section 10113 of the 2018 Farm Bill states that (c) Nothing in this subtitle shall affect or modify:
- (1) the Federal Food, Drug, and Cosmetic Act (21 U.S.C. 301 et seq.);
- (2) section 351 of the Public Health Service Act (42 U.S.C. 262); or
- (3) the authority of the Commissioner of Food and Drugs and the Secretary of Health and Human Services- ‘‘(A) under- ‘‘(i) the Federal Food, Drug, and Cosmetic Act (21 U.S.C. 301 et seq.); or ‘‘(ii) section 351 of the Public Health Service Act (42 U.S.C. 262); or ‘‘(B) to promulgate Federal regulations and guidelines that relate to the production of hemp under the Act described in subparagraph (A)(i) or the section described in subparagraph (A)(ii).”
There is nothing unclear about this issue. The same 2018 Farm Bill that hemp manufacturing companies use to justify the legality of hemp and CBD products is the same bill that spells out the authority of the FDA in this matter.
The mission of the FDA is “to ensure the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices.” The agency also is responsible for “the safety and security of our nation’s food supply, cosmetics, dietary supplements, products that give off electronic radiation, and for regulating tobacco products.” Health or medical claims not supported by clinical proof will not be tolerated. An unsafe, unclean or untested product will also not be tolerated in the marketplace.
CBD Oil vs. Isolate

Then there is the matter of CBD as either a full spectrum oil vs. an isolate…Unlike marijuana flower which is a very popular product, hemp flower is very rarely sold at the retail level. Full spectrum oil is extracted from the plant, and depending on the solvent used, produces an oil with the same, or close to the same, naturally occurring chemicals from the plant. The oil therefore, includes all the cannabinoids present along with any terpenes, lipids or other compounds present in the plant. Full spectrum oil is a botanical extract and is a dark thick oil. Isolate is produced by separating the constituents of the full spectrum oil by molecular weights or boiling points to have very pure chemicals in the 95%+ purity range. CBD isolate is a white crystalline substance and bears the greatest resemblance to a synthetic raw material and at its purest form cannot be distinguished as coming from a plant in the dirt or a synthesized chemical. Epidiolex is produced from hemp isolate and was approved by the FDA as a pharmaceutical. Full spectrum hemp oil is a botanical extract, often as an ethanol extraction. Full spectrum oil bears the greatest resemblance to a botanical dietary supplement. It remains to be seen what the FDA will allow in the future.
Product Labeling
The FDA has made it abundantly clear in numerous warning letters issued to the cannabis industry that drug claims (articles intended for use in the diagnosis, cure, mitigation, treatment or prevention of disease) regarding CBD, oil or isolate, cannot be made without pharmaceutical approval of the Drug Facts (Epidiolex) lest there be enforcement consequence.

The labeling of other types of products are less clear. Dietary supplements are a category of foods with the FDA and as such both the labeling of dietary supplements and foods are dictated in 21 CFR 111, Food Labeling. Botanical dietary supplements frequently call out a chemical constituent within a particular botanical material or extract on the Supplement Facts Panel: Milk thistle seed extract containing standardized and labeled silymarin is such an example. Is this strategy acceptable for CBD with the FDA? What about “naturally occurring” CBD? Food claims are indicated in the Nutrition Facts, what can these be for CBD? Cosmetic product claims can only address articles intended to be applied to the human body for cleansing, beautifying, promoting attractiveness, or altering the appearance without affecting the body’s structure or functions. What is the purpose of CBD in a cosmetic?
FDA guidance would be very beneficial in all of these labeling areas, and there is hope. The FDA is promising public hearings this spring to discuss a path forward for having hemp food and dietary supplements. The FDA will ask for public comment and hopefully, there will be a lot of public comments provided to them. The public’s huge demand for CBD products will bear pressure on the FDA to at least listen and consider.
cGMPsRegulatory compliance will be difficult, and it will be expensive.
Those currently in the hemp manufacturing industry should pay attention and take the FDA seriously. If the FDA allows hemp products with CBD to be sold in the future, it will be the FDA who makes those regulations and those products will have to fit into an already existing FDA category: human food, animal food, dietary supplement, pharmaceutical or cosmetic. If you are a hemp product manufacturer, you must learn the applicable requirements for Current Good Manufacturing Practices (cGMPs) by hiring experienced FDA compliance personnel, and/or seeking out FDA regulatory consultants, to develop and implement a quality system accordingly:
- 21 CFR 117, Current Good Manufacturing Practice, Hazard Analysis, and Rick-Based Preventative Controls for Human Food
- 21 CFR 507, Current Good Manufacturing Practice, Hazard Analysis, and Rick-Based Preventative Controls for Food for Animals
- 21 CFR 111, Current Good Manufacturing Practice in Manufacturing, Packaging, Labeling, or Holding Operations for Dietary Supplements
- 21 CFR 210, Current Good Manufacturing Practice in Manufacturing, Processing, Packing, or Holding of Drugs; General
- 21 CFR 211, Current Good Manufacturing Practice for Finished Pharmaceuticals
- FDA Draft Guidance for Industry, Cosmetic Good Manufacturing Practice, June 2013
I believe in this industry and I am rooting for the pioneers who have taken all the risk thus far, but the level of denial of the FDA’s authority that I am hearing in the hemp industry community is disturbing to me because those companies will not manage the transition to a regulated future. Most don’t understand it and they don’t think it applies to them or their products. Regulatory compliance will be difficult, and it will be expensive. The hemp pioneers deserve to benefit from their labor and the risk they have taken. For those hemp product companies that do not think compliance is worth the effort or cost, there are many FDA-compliant human food, animal food, dietary supplement, pharmaceutical, or cosmetic companies that are waiting to take your business…
Editor’s Note: While Cannabis Industry Journal typically does not use the term ‘marijuana,’ the author here is speaking from a regulatory point of view and creates an important distinction. Peyton chose the word “marijuana” instead of “cannabis” because the FDA has chosen “cannabis” to refer to both marijuana and hemp.




This is the best article I have read on the FDA/CBD issue. Well done!
The FDA public hearing on May 31st was announced: Statement from FDA Commissioner Scott Gottlieb, M.D., on new steps to advance agency’s continued evaluation of potential regulatory pathways for cannabis-containing and cannabis-derived products.
https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm635048.htm?utm_campaign=040219_Statement_FDA%20announces%20new%20steps%20on%20cannabis-related%20products&utm_medium=email&utm_source=Eloqua
Can a company become compliant for cGMP if they source the CBD from another company and integrate that CBD isolate that was sourced into a topical base to make a final topical product?
It’s very nice to see. I would like to comment your post. You did a great work here. I am happy to share this post to my friends. Wish you good luck for your future blogs.