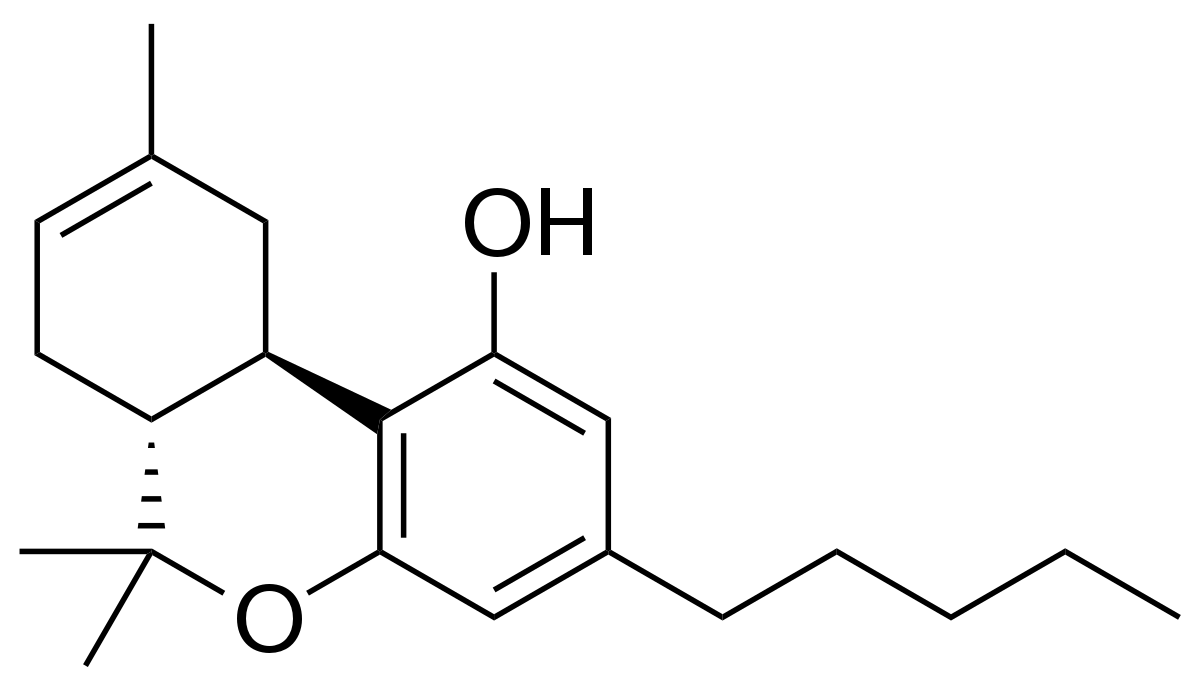
Natural cannabinoid distillates and isolates are hydrophobic oils and solids, meaning that they do not mix well with water and are poorly absorbed in the human body after consumption. Cannabinoid oils can be formulated into emulsions to form a fine suspension in water to improve bioavailability, stability and flavor. Vertosa is a cannabis infused ingredients company specializing in emulsion technologies. Their technology can be found in a range of CBD and THC containing beverages found on shelves today.
We spoke with Austin Stevenson, chief innovation officer at Vertosa, to learn more about emulsification technology and some of the challenges in testing cannabis infused beverages. Stevenson joined Vertosa in 2019 after spending time as a cannabis advisor at CanopyBoulder as an entrepreneur in residence. Prior to Vertosa, Stevenson ran the hemp and CBD analytical testing laboratory business unit for Eurofins.
Aaron Green: How did you get involved in the cannabis industry?
Austin Stevenson: I got involved in the cannabis industry nearly seven years ago, when I was an advisor to an accelerator in agriculture technology in Africa. I went to the MIT Innovation Laboratory, and I saw a whole bunch of farmers cultivating green leafy vegetables in the middle of the Kalahari Desert, which piqued my curiosity. I learned that it was all done via hydroponic indoor cultivation and freight containers. I got back to the US and put my detective hat on, and learned that it was really the cannabis industry that was driving innovation in terms of indoor and sustainable agriculture. At that point, I took it as an opportunity to dive in and started, again, as an advisor at an accelerator in Colorado. From there, I’ve been on the amazing cannabis journey.
Green: And how did you get involved with Vertosa?

Stevenson: I became an advisor at CanopyBoulder to a few software companies and got on the founding team there as well as at a few cultivation companies and other license types across the supply chain. Immediately before Vertosa, I ran the business unit for hemp and CBD testing at Eurofins, one of the world’s largest analytical chemistry laboratories, specializing in Ag Pharma. My clients were your traditional retailers: CVS, Kroger. Our team analyzed thousands, maybe hundreds of thousands of SKUs of infused products.
At one point I had to tell one of my clients at Eurofins, that all of their beverage SKUs were failing potency tests. Their supplements, OTC products, some of the confections, cosmetics, were all passing, but the beverages were failing potency testing. Cannabinoid ingredients were floating to the top, sinking to the bottom, even leaching into the can liners. It just wasn’t working, so we had to tell them that those beverages could not go to market. On this same day, I happened to run into my longtime friend and business partner in the industry (now Vertosa CEO) Ben Larson at a conference in Oakland, who was running the Gateway Incubator at the time, but had met our other partner and founder, Dr. Harold Han. Ben told me, “I have this PhD chemist, a surface chemist from BioRad. He’s been experimenting with techniques, taking cannabis oils and turning them into fast acting emulsions for beverages. I’d like for you to check it out because I’m considering building a business around this.” I said, “Alright, show me the technology. Let me take it back to the lab, analyze it, verify it, and then try it. See if it works.” Lo and behold, it did. I fell in love with the product. I saw the problem firsthand at my lab and now I saw a solution, so I knew that the next part of my cannabis journey would be to join Ben and Harold in building a business together focused on being the number one technology solving the problem of stability and potency for the infused beverage market.
Green: What is the core technology of Vertosa?
Stevenson: Our focus at Vertosa is being the best delivery mechanism for cannabinoids. That means that we have a portfolio of different technologies that we’re using to take cannabis oils and turn them into fast-acting liquid emulsions, as well as powder-based APIs. When we began, we were using nano-emulsification. We are using nanotechnology in the food space, with a few different methods for creating those nano-emulsions, to infuse a diverse range of different products – everything from seltzer waters to dealcoholized wines and teas.
Green: So, it’s a portfolio of products with the basic idea of encapsulating the oil into smaller components. Can you highlight some of the challenges when you were first developing the product with testing? My assumption is that it was relatively new for testing labs. How did you support method development with them so that you are accurately reporting cannabinoid content?
Stevenson: The biggest problem that we faced at Vertosa is that there’s no one size that fits all. The chemistry of an infused seltzer water is different than the chemistry of a dealcoholized wine. The reason is because, quite literally, the ingredients are different. They’re different products. When we’re making the emulsions for these beverages, all the ingredients have to be compatible – the ingredients in the emulsion as well as the ingredients in the beverage. We’ve had to design a portfolio of different emulsions for different beverage types to ensure compatibility in any scenario, otherwise there could be instability, causing separation between the emulsion and the ingredients.
Additionally, we’ve seen challenges in the packaging type as well as the manufacturing techniques, specifically sterilization, thermal processing, chemical treatment, or the lack thereof. These three core variables (ingredients, packaging, and manufacturing technique) are where all the challenges in potency testing arise. For example, you have an infused beverage that is going to be packaged in an aluminum can. There is a polarity between cannabinoids and the can aligners that ultimately could create leaching, or an absorption type of effect.
![]() At Eurofins, we would see beverages that were supposed to contain CBD in the can but were testing at 0 milligrams, despite manufacturers confirming that they had added the CBD. All the CBD had been absorbed into the can liner. Our teams of method development chemists and management had learned to acid rinse the can liner so that we would be able to capture the cannabinoids and identify them. That was a step that we had to learn through trial and error, and we were able to bring this over and build upon this at Vertosa.
At Eurofins, we would see beverages that were supposed to contain CBD in the can but were testing at 0 milligrams, despite manufacturers confirming that they had added the CBD. All the CBD had been absorbed into the can liner. Our teams of method development chemists and management had learned to acid rinse the can liner so that we would be able to capture the cannabinoids and identify them. That was a step that we had to learn through trial and error, and we were able to bring this over and build upon this at Vertosa.
Here at Vertosa, the biggest challenge in the lab currently is that there aren’t consistent methods for analyzing beverages. Every lab has different standards, and the instrumentation hasn’t always been calibrated. To ensure that these low dose beverages are measured properly, you have an accurate LOQ to identify the cannabinoid content. Part of the challenge is that the analytical chemistry community has only started to collaborate here recently, literally in the last few months as the AOAC made a call to action for methods for beverage.
At Vertosa, we’ve had to work together with the labs and ask if they have a method for developing beverages. It’s a three-step approach: we send a lab the oil, the emulsion, and the finished product, and ensure that the accurate cannabinoid profile is being diluted across the entire chain to make sure that each step the instrumentation has been calibrated the correct way. We want to make sure that they calibrate it into the HPLC and that the correct cannabinoid profile is always consistent in the finished product. It’s a lot of intimate hand-holding with the labs.
Green: So, you took it upon yourself to go out and get the methods validated, anticipating the need for finished goods testing with your customers and partners?
Stevenson: That’s right. From the beginning, we understood that the problems we are setting out to solve are consistent potency testing and accurate dosing. We wanted to be able to say confidently that when you work with us, you’re going to pass potency tests every time. And if you don’t, we’re going to uncover the reasons why.
For us, we have been able to provide that consistent and reliable ingredient. And yes, there’s been stumbles along the way, but those stumbles are the learnings that make us better. In the beginning, we had just one formula but the chemistries of different beverages vary too much for that to work. We also know that packaging type and manufacturing processes play a role. So, we now have a portfolio of different emulsions, such as conventional, natural, and organic, that can work with any given varibale and that have verifiable potency.
We anchor ourselves to the promise that our clients will pass potency, because that’s the biggest problem most brands have.We know the ingredients inside and out – knowing how heat plays a role, how polyphenols play a role, how oxygen plays a role, and helping the labs and our brand partners succeed while minimizing all the risk and pain that they go through with failed potency. You’d be surprised how many people are using the wrong product in formulation. A new client will come to us frustrated after adding CBD isolate powder to their beverage and seeing it fail potency tests. That’s where we’re able to come in and correct the course.
Green: Someone comes in with a magic wand. What do they solve for you?
“Efficacy research is the most interesting aspect of industry research to me.”Stevenson: If I had a magic wand, I would use it to accelerate efficacy research to validate and verify specific cannabinoids/terpene formulas for targeted effects. In other words, I’d love to have a peer-reviewed, scientifically validated cannabis formula for any desired effect, like anxiety or pain relief, aid in sleep, or increased energy, for example. At Vertosa, we’re currently investing in third party academic research to empower our clients with validated information; however, it takes a lot of time, money, and effort conducting research and clinical trials. It’s a long but essential and beneficial process!
Green: What trends are you following in the industry?
Stevenson: In the world of edibles and ingestibles, I’m extremely interested in exploring onset times and bioavailability technologies, as well as trends in ingredients. More of our clients are interested in rapid onset times so that consumers feel the effects within minutes of consumption, removing some of the stereotypical hesitation around edibles and wondering when “it’ll hit.” It’s also fascinating to explore and integrate minor cannabinoids as well as active and functional ingredients and how they interact together in an ingestible.
I’m also extremely interested in keeping up with changing regulatory policy around consumption lounges and access in recently recreational states. Open consumption lounges are a fantastic solution to further normalizing cannabis usage and decentralizing alcohol in our culture, as consumer behavior is increasingly reflecting a move away from alcohol towards more health-conscious choices.
Green: What are you most interested in learning about?
Stevenson: Efficacy research is the most interesting aspect of industry research to me. Most of us cannabis professionals are passionate about the plant, and anecdotally know how cannabis can be used to improve quality of life. However, the scientific and academic community needs to see hard evidence. As we build the industry in a post-prohibition era, there is more access to research grants to evaluate the efficacy and safety of cannabis. The National Institute of Health (NIH) has identified four (4) key areas of cannabis research eligible for grant funding: (1) cannabinoid research (2) cannabidiol research (3) endocannabinoid system, ECS research, and (4) therapeutic effects of cannabinoids. It’s the latter two, ECS and therapeutic effects, that really spark my curiosity. At VERTOSA, we’re spending a lot of time and resources with our Scientific Advisory board to help accelerate this research, and I’m personally excited about the forthcoming discoveries we make, which will help our entire industry grow and thrive!