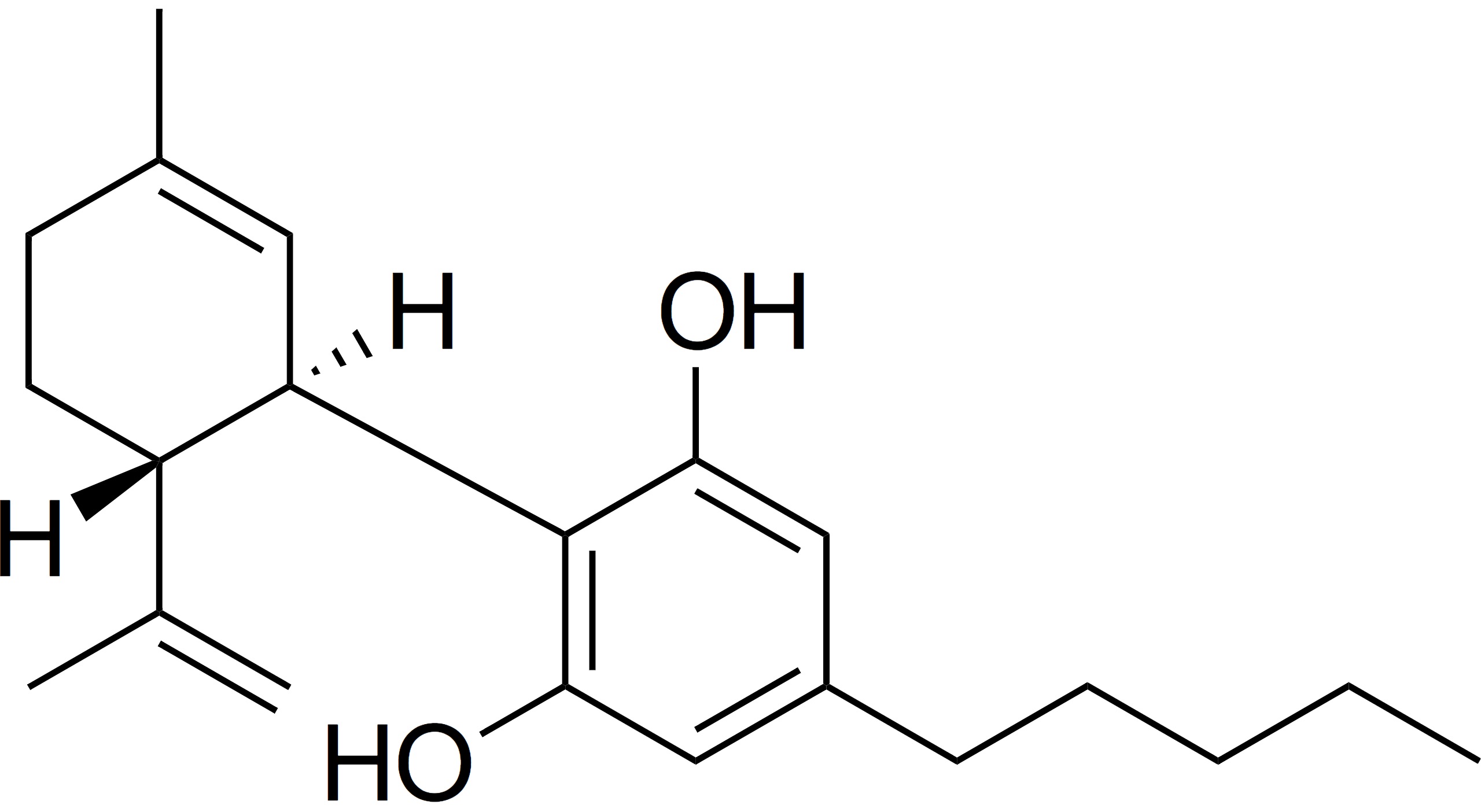
If you were at Davos this year, you heard alot about CBD. The cannabinoid will again be a headliner in business analysis and bottom line reports this year. But as the market matures, globally, what is the real temperature of the industry? And how fast will regional hiccups resolve?
Regulatory Issues Are In The Room
From the US state markets to the EU, hemp is coming into its own, even though almost everyone also refers to it as CBD (cannabidiol).
 In the United States, things are even more murky because of a lack of federal reform and the individual rules and regs of existing state markets. To an extent, the market is being “federalized” on the testing front (see ISO for example) and GMP (at the federal pharmaceutical level), producers are beginning to be able to get certified on a global scale. However, the vast majority of the U.S. market is not anywhere close to the regulatory muster now required of even the most-humble commercial hemp farmer anywhere in the EU.
In the United States, things are even more murky because of a lack of federal reform and the individual rules and regs of existing state markets. To an extent, the market is being “federalized” on the testing front (see ISO for example) and GMP (at the federal pharmaceutical level), producers are beginning to be able to get certified on a global scale. However, the vast majority of the U.S. market is not anywhere close to the regulatory muster now required of even the most-humble commercial hemp farmer anywhere in the EU.
In Europe, the entire cannabis discussion is already far more defined, and as a result, very much likely to set the rulebook globally, especially as so many people want to import here. And this is going to be a bugbear for the next two years. The rules on EU Bio for starters, are still in flux. And where this ties into GMP downstream, those who brave such waters are in for choppy seas for the time being.
Tie this into Novel Food, and this is an area right now that should only be charted by the most experienced navigators, and not just using the stars.
The Battle Is On – Both On The High Seas And The High Streets
For all the desire to bring “whole plant” into the room, (in other words recreational cannabis and medical cannabis with the THC still attached), CBD fever at least has spread in Europe faster than any pending flu epidemic from China.
There are positives and negatives that come with this discussion. Namely, the ever pounding need to commercialize the legal industry and remove all Drug War stigma and barriers from the discussion.
CBD-only legalization is also a powerful answer to those who claim that if CBD is legit, then the police will not chance busting people, no matter how much THC is or is not in the offending substance in question.
These are also the same people frequently who also have a stake in some level of the industry as it legalizes. And this is also where some of the fiercest battles for regulatory control and definition have also begun to happen.

Where they have come to a head (see Italy), it appears that governments are indeed reconsidering the whole “insurance” if not “home grow” discussion. Not to mention, as a result, recreational after that. The conversation in Italy, of all places, right now, is a good indication of this trend. It is a conservative country in every way, yet it is the first to not only cancel a government controlled monopoly license, but also the largest country in Europe to again tinker with limited home grow of cannabis plants.
Ironically this is also the place where the most dedicated “CBD revolutionaries” have also hit. In places like the UK right now, the lack of appetite for EU regulatory control generally (see Brexit) has resonated, particularly with a pro cannabis crowd sick and tired of more delay on a topic whose day in the sun has finally come. If not more government wobbles on discussion on the medical side (see the recent NHS decision to ignore cannabinoids and chronic pain).
In other places like Europe however, and this certainly showed up at Davos, CBD is a hardy foot soldier if not cannaguerilla from the hills that is beginning to chalk up discussions if not yet wide-ranging sovereign victories.
This is absolutely clear to see in places like the African market (and Lesotho is about to become a hot ticket globally if not within the African continent). Indeed, the first seeds were sown several years ago).
Yes, it is ridiculous that CBD is being banned. And it is also obvious that governments are unwilling to be bankrupted over medical cannabis of any kind or THC concentration, and know they must also seek other ways to deal with the issue.
CBD, in other words, is a kind of Che Guevara that is going to take down a few of the established orders in this revolution that is now global. And for that very reason, taking on a character if not place at the table all of its own.