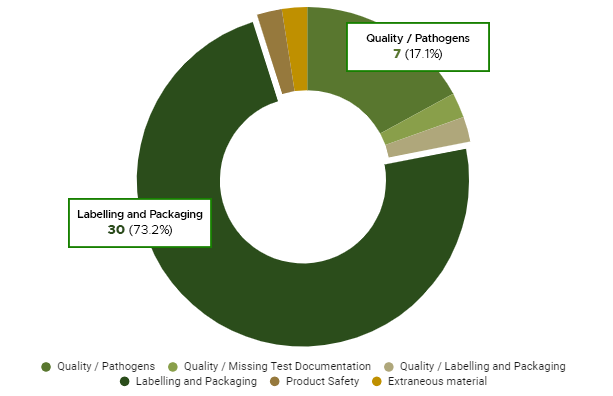
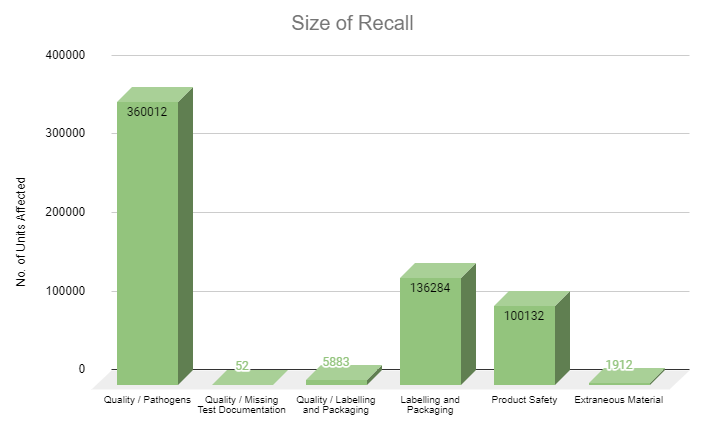
Cannabis brands are facing a proverbial fork in the road: determining whether their product evolves into a luxury consumable or affordable agricultural commodity. While it is reasonable to assume the cannabis market space will organically grow into a luxury goods industry such as wine and spirits, the luxury brands that serve as the foundation of these markets were built over years of engagement between consumers, connoisseurs and producers. If cannabis companies want to successfully market their products as luxury items, a concerted effort towards well-defined, consumer-accessible branding is required.
The first step towards evolving a cannabis brand towards luxury is overcoming the fixation on cultivar identity. Unregulated cultivar naming currently impedes creativity and craftsmanship, disrupting brands and salespersons’ abilities to clearly communicate strain aesthetics.
The good news is the alcohol, coffee and consumer packaged food (CPG) industries have done most of the heavy lifting, paving the way with robust sensory science and analytical approaches to product characterization. Cannabis stakeholders need only adapt their tools and apply them to cannabis with similar intention.
Research suggests that aroma is one of the strongest predictors of positive consumption experience. As adult use consumers become familiar with current product offerings and increasingly legal availability, they will seek products that consistently yield the best experience. The most successful brands will be those that most effectively communicate that experience and then deliver it. The status quo – describing aroma using strain names, top terpenes or THC content – is not effective at harmonizing a brand’s promise with consumer experience.
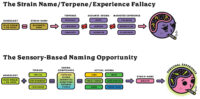
The conventional cannabis product branding approach leaves to be desired a tremendous opportunity to characterize cultivars (Figure 1). Sensory science, the discipline used to evoke, measure, analyze and interpret reactions to materials through human perception, has been used for decades to characterize CPGs from skin lotion to washing machines. Adapting these well-established techniques for use in cannabis can be challenging, but it is certainly worth the investment.
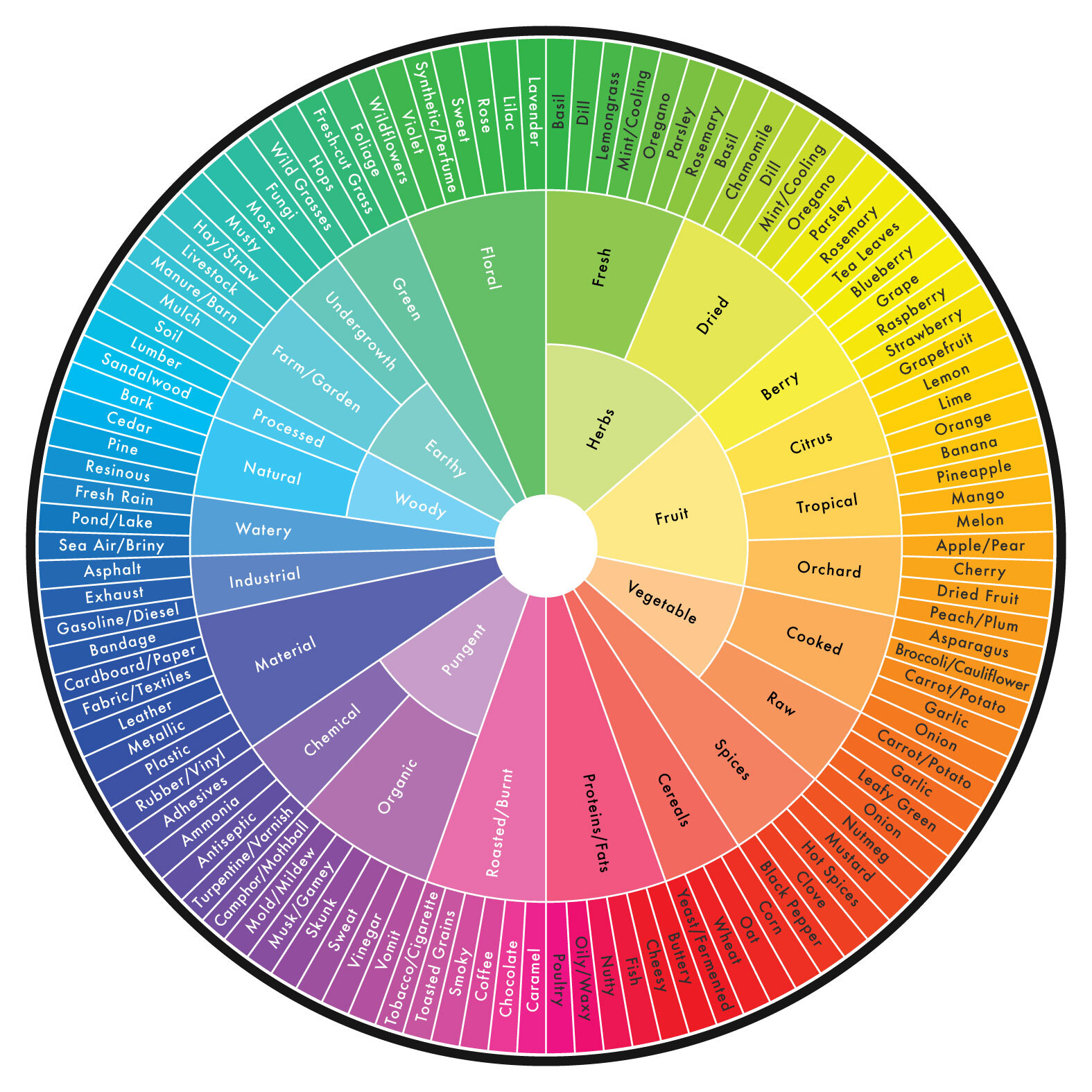
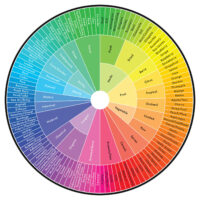
These shifts in the cannabis industry have already begun to occur. I recently was the principle investigator on a white paper that presented a novel cannabis aroma wheel derived from aroma descriptors and a panel of trained sensory experts. In the study, sensory scientists evaluated randomly sampled cannabis flower over a period of several months. The researchers combined qualitative focus panels, literature review and formal blinded sensory tests to develop a comprehensive lexicon that served as a tool for trained evaluators to characterize cannabis aroma. This novel and robust tool (Figure 2) was designed to be used by trained panels to characterize cannabis aroma, is freely available and is just the beginning of a collective development of a data-driven cannabis lexicon.
Much like the World Coffee Research Lexicon referenced here, the Cannabis Aroma Lexicon is a tool with a specific purpose: collecting an objective description of the product aroma. It is a living document that will grow along with the industry. In the future, we may have lexicons to describe more than just aroma. Tactile and appearance sensory attributes of cannabis will gradually be defined by sensory scientists, presenting more opportunities for deep craftsmanship in the cannabis industry.
The role that dispensaries play in adoption of standardized quality metrics is critical. The product features that position cannabis to be a craft product warrant the presence of a third-party expert to translate and guide consumers during the purchasing process. It’s intuitive to ask a waiter to recommend a pairing (i.e., a dry red wine to pair with a seafood dish), given the trust that consumers put in the restaurant to understand the properties of the food they are serving. Dispensaries have thus far filled the service structure role for cannabis, but the vast amount of unknowns regarding the physiological and sensorial effects of cannabis have resulted in inconsistent experiences that leave something to be desired in terms of consumer trust.
Application of sensory science in cannabis is an unparalleled opportunity for brands to build consumer trust and differentiate their products in a sea of strain names and high potency flower. Cultivars that can be established as measurably aromatic in a specific character can leverage that aroma profile to add significant value to the product. For example:
- Cultivar name can be aligned with the perceived aroma (e.g., garlic is not bad, but expecting tropical and getting garlic can harm consumer trust).
- Product catalog can be consolidated and optimized to avoid sales cannibalization by growing specific products to meet consumer group’s needs.
- Guesswork is removed from breeding by measuring when a product is meeting sensory goals and ensuring it doesn’t drift over time.
- Demonstrating transparency will win over ethical consumers. Utilizing controlled, blinded studies to profile aromas will add value to ethics-minded consumers.
- Becoming a leader in connoisseurship. In the forefront of this shift in branding, one can position a brand to be ahead of the competition.
- Elevating the dispensary experience. By utilizing aroma profiling, products can be more easily sold by budtenders and salespeople.
- Opening doors to new application types (e.g., seasonal, occasion centered or geographically unique cultivars).
These are just some of the ways that aroma characterization will differentiate products and simultaneously contribute to cannabis brands’ ability to communicate craftsmanship and the maturity of the industry as whole. Not only will adoption of robust sensory-based branding improve the consumer experience by providing a means to compare products to one another, it will promote adoption of good manufacturing practices that simultaneously improve the quality and safety of cannabis products. Without consumer-accessible quality metrics, brands have little incentive to produce products of elevated quality and are conversely incentivized to prioritize quantity and meet minimum regulatory requirements.
Importantly, cannabis businesses will use this tool to adapt to an inevitable industry-wide shift towards connoisseurship and application of robust sensory science. While it may be challenging to shake off the “bad habits” that currently plague many brands, cannabis has significant potential as a luxury good. Consumers are eager for a better cannabis experience from purchase to consumption. How will your brand use sensory profiling to expand or evolve product offerings to succeed in a cannabis market full of luxury brands and what steps will you take now to prepare?