With the cannabis industry growing rapidly, laboratories are adapting to the new market demand for medical cannabis testing in accordance to ISO/IEC 17025. Third-party accreditation bodies, such as Perry Johnson Laboratory Accreditation, Inc. (PJLA), conduct these assessments to determine that laboratories are following relevant medical cannabis testing standard protocols in order to detect potency and contaminant levels in cannabis. Additionally, laboratories are required to implement and maintain a quality management system throughout their facility. Obtaining accreditation is a challenge for laboratories initially going through the process. There are many requirements outlined in the standard that laboratories must adhere to in order to obtain a final certificate of accreditation. Laboratories should evaluate the ISO 17025 standard thoroughly, receive adequate training, implement the standard within their facility and conduct an internal audit in order to prepare for a third-party assessment. Being prepared will ultimately reduce the number of findings detected during the on-site assessment. Listed below is research and evidence gathered by PJLA to determine the top ten findings by clause specifically in relation to cannabis testing laboratories.
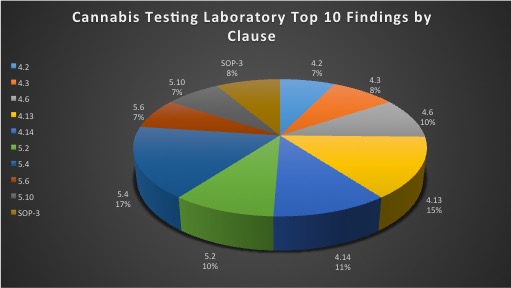
4.2: Management System
- Defined roles and responsibilities of management system and its quality policies, including a structured outline of supporting procedures, requirements of the policy statement and establishment of objectives.
- Providing evidence of establishing the development, implementation and maintenance of the management system appropriate to the scope of activities and the continuous improvement of its effectiveness.
- Ensuring the integrity of the management system during planned and implemented changes.
- Communication from management of the importance of meeting customer, statutory and regulatory requirements
4.3: Document Control
- Establishing and maintaining procedures to control all documents that form the management system.
- The review of document approvals, issuance and changes.
4.6: Purchasing Services and Supplies
- Policies and procedures for the selection and purchasing of services and supplies, inspection and verification of services and supplies
- Review and approval of purchasing documents containing data describing the services and supplies ordered
- Maintaining records for the evaluation of suppliers of critical consumables, supplies and services, which affect the quality of laboratory outputs.
4.13: Control of Records
- Establishing and maintaining procedures for identification, collection, indexing, access, filing, storage and disposal of quality and technical records.
- Providing procedures to protect and back-up records stored electronically and to prevent unauthorized access.
4.14: Internal Audits
- Having a predetermined schedule and procedure for conducting internal audits of its activities and that addresses all elements that verify its compliance of its established management system and ISO/IEC 17025
- Completing and recording corrective actions arising from internal audits in a timely manner, follow-up activities of implementation and verification of effectiveness of corrective actions taken.
5.2: Personnel
- Laboratory management not ensuring the competence and qualifications of all personnel who operate specific equipment, perform tests, evaluate test results and sign test reports. Lack of personnel undergoing training and providing appropriate supervision
- Providing a training program policies and procedures for an effective training program that is appropriate; identification and review of training needs and the program’s effectiveness to demonstrate competence.
- Lack of maintaining records of training actions taken, current job descriptions for managerial, technical and key support personnel involved in testing
5.4: Test and Calibration Methods and Method Validation
- Utilization of appropriate laboratory methods and procedures for all testing within the labs scope; including sampling, handling, transport, storage and preparation of items being tested, and where appropriate, a procedure for an estimation of the measurement of uncertainty and statistical techniques for analysis
- Up-to-date instructions on the use and operation of all relevant equipment, and on the handling and preparation of items for testing
- Introduction laboratory-developed and non-standard methods and developing procedures prior to implementation.
- Validating non-standard methods in accordance with the standard
- Not completing appropriate checks in a systematic manner for calculations and data transfers
5.6: Measurement Traceability
- Ensuring that equipment used has the associated measurement uncertainty needed for traceability of measurements to SI units or certified reference materials and completing intermediate checks needed according to a defined procedure and schedules.
- Not having procedures for safe handling, transport, storage and use of reference standards and materials that prevent contamination or deterioration of its integrity.
5.10: Reporting the Results
- Test reports not meeting the standard requirements, statements of compliance with accounting for uncertainty, not providing evidence for measurement traceability, inaccurately amending reports.
SOP-3: Use of the Logo
- Inappropriate use of PJLA’s logo on the laboratories test reports and/or website.
- Using the incorrect logo for the testing laboratory or using the logo without prior approval from PJLA.



